balloon flight
aviation
Introduction
 passage through the air of a balloon that contains a buoyant gas, such as helium or heated air, for which reason it is also known as lighter-than-air free flight. Unmanned balloons have been used to carry meteorological instruments and may be radio-controlled. Manned balloons have a basket, or gondola, attached below the balloon for the pilot and passengers. A simple harness or boatswain's chair has become popular for solo flights. By adjusting the ascent and descent of a balloon through the air, a pilot can take advantage of available winds to guide the course of the balloon over the surface of Earth. This element of control, or the lack of it, is the hallmark of sport ballooning.
passage through the air of a balloon that contains a buoyant gas, such as helium or heated air, for which reason it is also known as lighter-than-air free flight. Unmanned balloons have been used to carry meteorological instruments and may be radio-controlled. Manned balloons have a basket, or gondola, attached below the balloon for the pilot and passengers. A simple harness or boatswain's chair has become popular for solo flights. By adjusting the ascent and descent of a balloon through the air, a pilot can take advantage of available winds to guide the course of the balloon over the surface of Earth. This element of control, or the lack of it, is the hallmark of sport ballooning.The first untethered manned balloon ascent took place on Nov. 21, 1783, when two Frenchmen climbed into a wicker basket suspended from the base of a beautifully decorated, paper-lined cotton balloon. The balloon, filled with air heated by burning straw, carried the men aloft for a little more than 20 minutes over Paris. Witnessing this ascension were Louis XVI, members of the French Academy of Sciences (Sciences, Academy of), and multitudes of the public, including the American inventor and statesman Benjamin Franklin (Franklin, Benjamin). This event left a profound impression on the world of the 18th century: Men had actually flown! Since that time, the field of flight has been taken over by airships, gliders, airplanes, helicopters, and even rockets and spacecraft, but balloons continue to be used for recreation, competitive sport, and scientific exploration.
Hot-air balloons may be used for short flights at low altitudes or taken on “long jumps,” using stronger winter winds to travel hundreds of kilometres at altitudes of up to about 3 km (2 miles). Gas balloons can stay aloft for several days and travel a thousand kilometres or more. Indeed, combination hot-air and gas balloons have crossed continents and oceans and even circled the globe. For scientific research, special gas balloons can float in stable conditions for days or even months at a time, carrying instrument payloads through the upper reaches of the stratosphere.
The Fédération Aéronautique Internationale (Féderátion Aéronautique Internationale) was founded in France in 1905. This nongovernmental organization maintains records for manned flights from balloons to spacecraft, as well as records for flights of model aircraft, unmanned aerial vehicles, and sporting events. In addition, various national aeronautics organizations, such as the Balloon Federation of America and the British Balloon and Airship Club, maintain ballooning records. Airworthiness and operating criteria are controlled in the United States by the Federal Aviation Administration (FAA). FAA regulations for ballooning are generally used by all countries, with only minor local variations.
Elements of balloon flight
The three basic principles of buoyancy were discovered by the ancient Greek mathematician and inventor Archimedes, the 17th-century British natural philosopher Robert Boyle (Boyle, Robert), and the 18th-century French physicist Jacques-Alexandre-César Charles (Charles, Jacques-Alexandre-César):
● Archimedes' principle (3rd century BC), which states that any body completely or partially submerged in a fluid (gas or liquid) at rest is acted upon by an upward, or buoyant, force the magnitude of which is equal to the weight of the fluid displaced by the body;
● Boyle's law (1662), which states that the pressure of a given quantity of gas varies inversely as its volume at constant temperature; and
● Charles's law (1787), which states that the volume occupied by a fixed amount of gas is directly proportional to its absolute temperature, if the pressure remains constant.
A balloon can carry the difference between its weight (including its enclosed gas) and the weight of the air that it displaces. Nine cubic metres (1,000 cubic feet) of hydrogen weighs about 2.2 kg (5 pounds), the same volume of helium weighs about 4.5 kg (10 pounds), methane 18 kg (40 pounds), and hot air, at normal hot-air balloon operating temperatures, 22.5 kg (50 pounds). Thus, the lifting force of a chosen gas at low altitudes can be obtained by subtracting its weight from the typical weight of the same volume of air (about 34 kg, or 75 pounds, in this example).
Because the atmosphere is compressed by its own weight, it is less dense at higher altitudes. At 3,600 metres (about 12,000 feet) the atmosphere is approximately two-thirds as dense and so will provide two-thirds the buoyancy. This effect continues progressively, so that at 15,000 metres (50,000 feet) it is only one-tenth as dense, at 30,000 metres (100,000 feet) one-hundredth, and at 45,000 metres (150,000 feet) one-thousandth. In order to carry the same load at an altitude of 50 km (30 miles) as at sea level, a balloon would have to be 1,000 times as big and yet weigh the same (that is, keeping everything constant except the balloon's volume).
The buoyancy of a hot-air balloon is controlled by heating the air in the balloon or by changing the amount of ballast (extra weight). The buoyancy of a gas balloon is controlled by changing the amount of gas in the balloon or the amount of ballast. Tiny changes in one of these components can force dramatic changes in a balloon's flight. Just a one- or two-degree change in temperature in a hot-air balloon, a few grams of ballast dropped, or a tiny release of gas will make a balloon ascend or descend accordingly. On the other hand, in violent maneuvers (such as during a storm), whole bags of sand or great blasts of heat from a balloon's burner may be required for a proper correction. To help in rapid atmospheric cooling, modern hot-air balloons also have very large hot-air release vents in the form of a parachute that can seal and unseal an opening in the top of the balloon.
Changing the altitude of a balloon will permit it to follow different air currents. A 20- or 30-degree difference in wind direction usually occurs in the first few thousand metres of altitude, but a full circle of wind directions (“box winds”) can also occur. Albuquerque, N.M., is famous for its box winds, which can be used to climb and descend back to the original launch site. If there is only a simple and stable wind pattern, no additional control is possible. With superior weather monitoring, use of the Global Positioning System ( GPS), and radio or satellite communication, remarkable flight control is now possible.
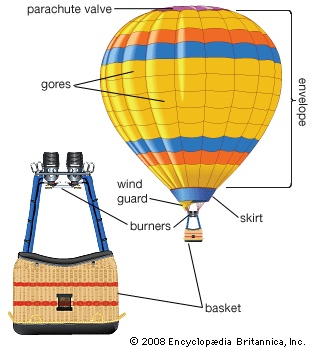 Early hot-air balloons burned straw and alcohol spirits for fuel, though by 1900 these fuels had been replaced with petroleum. Compressed liquefied propane is used almost exclusively today. Hot-air balloon burners use vaporizing coils to preheat the fuel for efficient combustion. Most of these coils are made of stainless steel, but copper coils also work adequately. The burners are mounted, often on gimbals, on the suspension concentration ring between the basket and the mouth of the balloon. Most systems have redundant burners to compensate for problems with defective controls or contaminated fuel. A particularly notorious early problem was leaking seals on control valves.
Early hot-air balloons burned straw and alcohol spirits for fuel, though by 1900 these fuels had been replaced with petroleum. Compressed liquefied propane is used almost exclusively today. Hot-air balloon burners use vaporizing coils to preheat the fuel for efficient combustion. Most of these coils are made of stainless steel, but copper coils also work adequately. The burners are mounted, often on gimbals, on the suspension concentration ring between the basket and the mouth of the balloon. Most systems have redundant burners to compensate for problems with defective controls or contaminated fuel. A particularly notorious early problem was leaking seals on control valves.A variety of materials has been used for the actual balloon, or envelope. cotton, nylon, and polyester are common for the envelopes of hot-air balloons. Cotton, having a poor weight-to-strength ratio, is only favoured for carnival “smoke” balloons. Although gas balloons have sometimes used rubberized cotton, modern sport gas balloons use urethane-coated nylon. Balloons for high-altitude research are generally made of polyethylene or polyester film. When a scientific payload is attached to the bottom point of a modern balloon, the envelope, if given overall excess material circumferentially, will form the shape of an acorn squash. This is a result of the natural stresses on the “skin” caused by the tension from the payload and the varying internal forces of the gas, which depend on the elevation inside the balloon. Additional excess material in the horizontal dimension will only result in loose wrinkles. Less horizontal fullness will cause tight spots or even indentations. A large lightly loaded balloon will be very “fat.” The top will have a large flat or even indented area, and the bottom point will have an obtuse included angle. If the balloon envelope is only a small percentage of the total weight, the balloon will have more of a teardrop shape, and the bottom point will have an acute included angle. This family of cross sections is known as the natural shape.
In addition to the choice of envelopes, an even greater variety of systems for carrying the load or passengers has been used, ranging from a simple trapeze to the sealed environmentally controlled cabin of the stratosphere balloon. For sport ballooning, the traditional wicker basket, albeit with a stainless steel frame, is popular. Criteria for evaluation of a basket design should include toughness, energy absorption, and electrical resistance, but style and marketability are more often the governing factors.
Historical development
The Montgolfier brothers
Credit for the invention of ballooning goes to a pair of 18th-century brothers, Joseph-Michel and Jacques-Étienne Montgolfier (Montgolfier, Joseph-Michel and Jacques-Étienne) of Annonay, a small town just south of Lyon, France. According to one, possibly apocryphal, story, the brothers took inspiration from watching Joseph's wife's skirts as they billowed in the kitchen from the heat of a charcoal burner being used to dry laundry.
The Italian-born English scientist Tiberius Cavallo had recently demonstrated that Archimedes' principle is applicable to airborne objects by successfully floating hydrogen-filled soap bubbles. When he attempted to duplicate this with animal bladders, though, they proved too heavy to ascend. Fortunately, the provincial Montgolfiers were not constrained by laboratory techniques and created their hot-air balloon on a cruder and much larger scale. Because the area (and therefore the weight) of a balloon goes up by the square of its diameter and the volume (and therefore the lift) increases by the cube of its diameter, they succeeded handily. On June 4, 1783, they made a public demonstration in Annonay with a 10.5-metre (35-foot) diameter unmanned cloth-and-paper balloon, using the heat from a straw fire.
Charles and de Rozier join the race
 With the news from Annonay, French inventor Jacques-Alexandre-César Charles (Charles, Jacques-Alexandre-César), who knew that hydrogen was lighter than the hot-air smoke used by the Montgolfiers, realized that all he had to do to succeed was to make his balloon experiment on a larger scale. The first space race was on. On Aug. 27, 1783, Charles launched an unmanned varnished-silk hydrogen balloon from Paris. It was attacked and destroyed by local villagers when it landed near Gonesse some 15 km (9 miles) to the northeast. The Montgolfiers countered by launching a hot-air balloon carrying a sheep, a duck, and a rooster from Versailles on September 19 to determine if the animals could survive in the open air at higher altitudes. The first person at the landing site of the menagerie balloon was Jean-François Pilâtre de Rozier, who would become the first balloon pilot.
With the news from Annonay, French inventor Jacques-Alexandre-César Charles (Charles, Jacques-Alexandre-César), who knew that hydrogen was lighter than the hot-air smoke used by the Montgolfiers, realized that all he had to do to succeed was to make his balloon experiment on a larger scale. The first space race was on. On Aug. 27, 1783, Charles launched an unmanned varnished-silk hydrogen balloon from Paris. It was attacked and destroyed by local villagers when it landed near Gonesse some 15 km (9 miles) to the northeast. The Montgolfiers countered by launching a hot-air balloon carrying a sheep, a duck, and a rooster from Versailles on September 19 to determine if the animals could survive in the open air at higher altitudes. The first person at the landing site of the menagerie balloon was Jean-François Pilâtre de Rozier, who would become the first balloon pilot.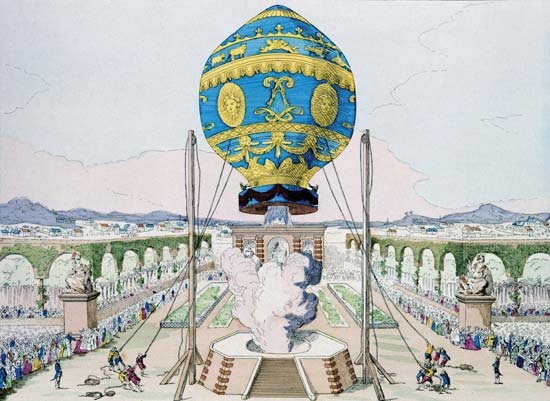 While Charles was designing—and having engineering brothers Marie-Noël and Anne-Jean Robert build—a larger hydrogen balloon that could carry him aloft, de Rozier was teaching himself to fly a hot-air balloon by first going up with a restraining rope. Before Charles could get his gas balloon ready, de Rozier and François Laurent, marquis d'Arlandes, persuaded the king to permit them to make the first manned free flight. On November 21 they went aloft over Paris. A little more than 20 minutes and 16 km (10 miles) later, they safely returned to Earth. Ten days later Charles made the first manned gas balloon ascension, accompanied by Marie-Noël Robert. On landing near Nesles, some 36 km (22 miles) away from the launch in Paris, Robert stepped out to let Charles make a second flight. The balloon ascended at a terrifying rate with Charles on the world's first solo free flight. The balloon finally leveled out at about 3,000 metres (10,000 feet), and he was able to bring it down safely.
While Charles was designing—and having engineering brothers Marie-Noël and Anne-Jean Robert build—a larger hydrogen balloon that could carry him aloft, de Rozier was teaching himself to fly a hot-air balloon by first going up with a restraining rope. Before Charles could get his gas balloon ready, de Rozier and François Laurent, marquis d'Arlandes, persuaded the king to permit them to make the first manned free flight. On November 21 they went aloft over Paris. A little more than 20 minutes and 16 km (10 miles) later, they safely returned to Earth. Ten days later Charles made the first manned gas balloon ascension, accompanied by Marie-Noël Robert. On landing near Nesles, some 36 km (22 miles) away from the launch in Paris, Robert stepped out to let Charles make a second flight. The balloon ascended at a terrifying rate with Charles on the world's first solo free flight. The balloon finally leveled out at about 3,000 metres (10,000 feet), and he was able to bring it down safely.The rip panel and drag rope
Most of the features of the classic free balloon were included in Charles's first machine. Important later additions were the rip panel, first used on April 27, 1839, by the American aeronaut John Wise, and the drag rope, invented about 1830 by the English aeronaut Charles Green (Green, Charles). A rip panel is an elongated section of the balloon that is lightly fixed in place and can be quickly ripped or pulled open at the moment of landing. It adds greatly to the safety of ballooning by making quick deflation possible. The drag rope, or compensator, serves two purposes. It is a long, heavy rope that trails the balloon in order to slow down the balloon's vertical and horizontal speed in landing operations before the basket touches down. If a landing is aborted, the rope is automatically recovered and can be used again. In areas without electrical power lines, balloons can “drag rope” for many kilometres at a time without having to drop sand or release gas.
The gas–hot air hybrid balloon
Within two years, de Rozier began thinking about flying across the English Channel. To compensate for the shortcomings of the two types of balloons, he combined a hydrogen envelope with a small hot-air envelope below it. Hydrogen provided the basic lift, while the hot-air balloon system allowed him to control his flight without having to constantly drop ballast or release gas. His balloon, christened Tour de Calais, was brilliantly decorated with artwork and metallic gilding. According to modern investigations, the metallic coating caused a static discharge that ignited the varnished envelope some 30 minutes after its launch from Boulogne on June 15, 1785. De Rozier and his passenger, Pierre-Jules Romain, died within minutes of the ensuing crash, becoming the first balloon fatalities. Despite this tragic failure, de Rozier's invention eventually succeeded in the ultimate transglobal balloon voyage two centuries later.
The three basic types of balloons (hot air, gas, and a gas–hot air hybrid) were, then, all invented at the very beginning. A fourth type, the superpressure balloon, which is kept at a constant volume, was proposed by French Gen. Jean Meusnier on Dec. 3, 1783, but not successfully built until stronger materials became available in the 1950s. See below Superpressure balloons (balloon flight).
Smoke and coal gas
 Smoke balloons, without onboard fire, became popular for fairs and exhibitions as parachutes were perfected. In particular, the standard grand climax of many celebrations at the turn of the 20th century was to have a trapeze artist ascend for hundreds of metres below a balloon belching black smoke before jumping from the trapeze to parachute back to Earth.
Smoke balloons, without onboard fire, became popular for fairs and exhibitions as parachutes were perfected. In particular, the standard grand climax of many celebrations at the turn of the 20th century was to have a trapeze artist ascend for hundreds of metres below a balloon belching black smoke before jumping from the trapeze to parachute back to Earth.The smoke was not just for dramatic effect; it was essential to retain heat, as no fire was carried onboard. Clean air cools rapidly in an ascending balloon, not only by radiation but also by the adiabatic process of expansion. The heat in the carbon particles is not affected by the change in atmospheric pressure during the ascent, so the smoke acts as a heat sink in addition to freshly sealing the porous muslin fabric typically used in such balloons.
On July 19, 1821, at the coronation in London of George IV, Charles Green made the first ascent in a balloon inflated with coal gas. He also made a historic flight on Nov. 7, 1836, from London to Weilburg, Duchy of Nassau (now in Germany), a distance of about 800 km (500 miles). Other great flights of the period included French aeronaut François Arban's September 1849 flight across the Alps and John Wise's 1,300-km (800-mile) flight from St. Louis, Mo., to Henderson, N.Y. Wise's flight, which was launched on July 1, 1859, was a test of the air currents for a proposed transatlantic attempt.
Military experiments and petroleum fuel
 Manned balloons have had only minimal military use, the Siege of Paris (Sept. 19, 1870–Jan. 28, 1871), during the Franco-German War, being a notable exception. Mail, carrier pigeons, and important individuals were transported in balloons built in the unused Paris railway stations, and the pigeons brought mail back.
Manned balloons have had only minimal military use, the Siege of Paris (Sept. 19, 1870–Jan. 28, 1871), during the Franco-German War, being a notable exception. Mail, carrier pigeons, and important individuals were transported in balloons built in the unused Paris railway stations, and the pigeons brought mail back.In 1903 the Rev. John M. Bacon invented the forerunner of the modern hot-air balloon in England. While coal gas was plentiful and inexpensive locally, expeditionary forces had severe logistic problems with producing hydrogen in the field or transporting heavy compressed-gas cylinders. Bacon promoted the concept of performing military observations with a hot-air balloon that would burn petroleum. His trials in the summer of 1903 were successful, but he did not pursue it further and his work went unnoticed in the ballooning community.
During the 1930s, attempts were made to utilize petroleum or propane fuels by German and Austrian pioneers. Their efforts were technically promising, but they did not replace the sport gas balloon. The philosophy of ballooning entailed long flights at considerable altitude. Hydrogen and coal gas were plentiful, inexpensive, and accepted fearlessly. Heavy cotton balloons with their cumbersome fuel systems were not suited to traditional ballooning routines. Even in England, where long-duration gas flights were not possible for fear of the sea, there was no interest.
Balloons reach the stratosphere
Unmanned sounding balloons for high-altitude scientific investigations were introduced in 1893, but manned ballooning was limited to moderate altitudes until the 1930s. In 1931 Swiss physicist Auguste Piccard (Piccard, Auguste) inverted a 1905 conception devised by him and his twin brother, Jean Piccard (Piccard, Jean-Felix), for a diving ship ( bathyscaphe). The 1931 invention consisted of a spherical aluminum pressure cabin and a 14,000-cubic-metre (500,000-cubic-foot) lightweight rubberized-cotton netless hydrogen balloon. This would make possible the first successful stratosphere flight. It carried Auguste and his assistant, Paul Kipfer, to 15,781 metres (51,775 feet) on May 27, 1931. Jean Piccard and his wife, Jeannette, went to 17,550 metres (57,579 feet) on Oct. 23, 1934, with a slightly larger duplicate that used a magnesium-alloy cabin. The official project was completed earlier when U.S. Navy Lieut. Comdr. Thomas G.W. Settle achieved a world-record flight of 18,665 metres (61,237 feet) in the same balloon on Nov. 20, 1933.
Jean and Jeannette Piccard's balloon had several novel advances, the most significant being the remote-control pyrotechnic ballasting system. Contrary to conventional designs, they used blasting caps and trinitrotoluene (TNT) to cut cords outside the sealed capsule.
The Piccard 17,550-metre flight was followed by longtime National Geographic magazine contributor Capt. A. Stevens (Stevens, Albert William) and Capt. Orville Anderson, both of the U.S. Army Air Corps, going to 22,065 metres (72,395 feet) on Nov. 11, 1935. The flight was sponsored by the National Geographic Society and the U.S. Army Air Corps. Stevens and Anderson used a 100,000-cubic-metre (3,700,000-cubic-foot) rubberized-cotton balloon carrying a large magnesium-alloy cabin. That balloon, the Explorer II, was seven times the size of Piccard's, but still with very similar fabric. The stress in the skin of the giant balloon was formidable, resulting in repeated failures. On one occasion the crew, this time including Maj. William E. Kepner, barely escaped by parachute.
plastic balloons
 Jean Piccard realized that the giant single-cell balloon had reached the end of its practical development. Larger balloons would require heavier fabric with diminishing returns. Small latex balloons were routinely carrying light loads to much greater heights, and Piccard postulated that with a cluster of these he could extend the limits of ballooning. Thomas H. Johnson of the Franklin Institute suggested using fewer but larger cellophane balloons. Piccard, working with Johnson, designed a netless film balloon that substituted a conical skin section for the suspension system. The payload was attached directly to the base of the cone. By 1937 Piccard and his students at the University of Minnesota, including Robert Gilruth (later head of Project Mercury (Mercury)), had flown one of these unmanned balloons about 1,000 km (600 miles), carrying an automatic ballast-releasing device and radio instrumentation.
Jean Piccard realized that the giant single-cell balloon had reached the end of its practical development. Larger balloons would require heavier fabric with diminishing returns. Small latex balloons were routinely carrying light loads to much greater heights, and Piccard postulated that with a cluster of these he could extend the limits of ballooning. Thomas H. Johnson of the Franklin Institute suggested using fewer but larger cellophane balloons. Piccard, working with Johnson, designed a netless film balloon that substituted a conical skin section for the suspension system. The payload was attached directly to the base of the cone. By 1937 Piccard and his students at the University of Minnesota, including Robert Gilruth (later head of Project Mercury (Mercury)), had flown one of these unmanned balloons about 1,000 km (600 miles), carrying an automatic ballast-releasing device and radio instrumentation.Piccard dreamed of a stratosphere flight with a cluster of film balloons, but there was concern that they would become tangled. To test the concept, he made a successful solo flight in the Pleiades with an ensemble of 92 latex balloons on July 18, 1937.
After World War II, General Mills, Inc., accepted a contract from the U.S. Office of Naval Research to advance the theory of ballooning. The DuPont Company's new polyethylene film was chosen for the envelope. Launches of individual test balloons were finally successful, but there was little faith that the complicated task of rigging 80 of these at once would work, and the project was abandoned. However, the plastic balloon had been created, and they constantly grew larger and more acceptable as polyethylene films improved.
The conversion of the seam tapes—from their original simple joining task to backup use over heat-welded seams and, finally, with reinforcing filaments, to the primary structural load-bearing factor—enabled further size increases and advanced reliability. Their use also enabled the abandonment of the load ring in the mouth of the balloon. This facilitated the development of the natural shape.
In December 1955 the U.S. Air Force established Project Man High to obtain scientific data on the stratosphere and to test equipment for exploring above Earth's atmosphere. Three pilots, Capt. Joseph W. Kittinger, Maj. David Simmons, and Lieut. Clifton M. McClure, in Manhigh I (June 2, 1957), Manhigh II (Aug. 19, 1957), and Manhigh III (Oct. 8, 1958), respectively, each ascended to about 30 km (19 miles) aboard a single-cell plastic balloon. Unmanned flights, generally carrying scientific research payloads of more than 2,250 kg (5,000 pounds), have reached altitudes above 42 km (26 miles) with balloons as big as 1,000,000 cubic metres (some 40,000,000 cubic feet).
Superpressure balloons
 polyester film at a tensile strength of 1,400 kg per square cm (20,000 pounds per square inch)—compared with polyethylene at a tensile strength of about 40 kg per square cm (600 pounds per square inch)—finally made it possible to produce superpressure balloons, which do not expand or contract as the enclosed gas heats up or cools down. A series of contracts were awarded to the G.T. Schjeldahl Company by the U.S. Air Force in the late 1950s to develop polyester balloons. After repeated failures, Donald Piccard (son of Jean and Jeannette Piccard) was assigned the project. He theorized that the failures were caused by the self-destructive tendencies of the stiff film. By laminating two layers of very thin Mylar, he produced a more flexible film that resulted in the first successful superpressure balloon. These balloons have been used by the U.S. National Center for Atmospheric Research to carry instrumentation aloft for months at a time, continually circumnavigating Earth.
polyester film at a tensile strength of 1,400 kg per square cm (20,000 pounds per square inch)—compared with polyethylene at a tensile strength of about 40 kg per square cm (600 pounds per square inch)—finally made it possible to produce superpressure balloons, which do not expand or contract as the enclosed gas heats up or cools down. A series of contracts were awarded to the G.T. Schjeldahl Company by the U.S. Air Force in the late 1950s to develop polyester balloons. After repeated failures, Donald Piccard (son of Jean and Jeannette Piccard) was assigned the project. He theorized that the failures were caused by the self-destructive tendencies of the stiff film. By laminating two layers of very thin Mylar, he produced a more flexible film that resulted in the first successful superpressure balloon. These balloons have been used by the U.S. National Center for Atmospheric Research to carry instrumentation aloft for months at a time, continually circumnavigating Earth.Manned superpressure balloons have had some success but have not yet been able to carry on through diurnal heating cycles.
Modern hot-air balloons
 A small group of engineers under Wes Borgeson at General Mills developed a polyethylene hot-air balloon with a propane burner that was successfully flown by Tom Olson and later by Paul (“Ed”) Yost perhaps as early as 1955. Yost, then at Raven Industries, made the first publicized flight of the modern hot-air balloon in 1961 at Bruning, Neb. The balloon, developed for “silent entry” (military) use, was soon found to be unsuited for covert operations because of the noise and light from the burners, and the classified project was apparently abandoned. Although these balloons proved unsuitable for military use, Mark Semich (Semco) and Donald Piccard (Don Piccard Balloons) pursued their American manufacture for sport service.
A small group of engineers under Wes Borgeson at General Mills developed a polyethylene hot-air balloon with a propane burner that was successfully flown by Tom Olson and later by Paul (“Ed”) Yost perhaps as early as 1955. Yost, then at Raven Industries, made the first publicized flight of the modern hot-air balloon in 1961 at Bruning, Neb. The balloon, developed for “silent entry” (military) use, was soon found to be unsuited for covert operations because of the noise and light from the burners, and the classified project was apparently abandoned. Although these balloons proved unsuitable for military use, Mark Semich (Semco) and Donald Piccard (Don Piccard Balloons) pursued their American manufacture for sport service.Yost's hot-air balloon, using strong and durable nylon fabric instead of gossamer polyethylene, did not use load tapes. While load tapes had been an important factor in the success of film balloons, they were considered unnecessary for fabric balloons. However, with the growth of sport ballooning, a longer life and a safer design was required. In 1964 Donald Piccard adopted the full-length load tapes found on plastic balloons for fabric balloons. Coincidentally, this afforded the opportunity for his invention of the bulbous gore, or pumpkin-shaped balloon.
American aeronaut Tracy Barnes adapted a venting system used in parachutes to make the most important advance in safety and control of hot-air balloons since the rip panel. Barnes's parachute top has also been used in gas balloons. His novel three-corner basket and three-point suspension distinguish his balloons from the commonplace.
Hot-air ballooning
Recreation and sport
 Hot-air balloons are commonly used for recreational purposes. In addition to quiet morning or afternoon flights drifting cross-country to enjoy the view, many balloonists enjoy competitive sporting events and attempting to set new records. A balloonist may fly alone in the basket or carry several passengers. Often several balloons meet to launch together without any competitive goals. Individual flights generally last from one to three hours and may go several kilometres, though they often land very close to the take-off point.
Hot-air balloons are commonly used for recreational purposes. In addition to quiet morning or afternoon flights drifting cross-country to enjoy the view, many balloonists enjoy competitive sporting events and attempting to set new records. A balloonist may fly alone in the basket or carry several passengers. Often several balloons meet to launch together without any competitive goals. Individual flights generally last from one to three hours and may go several kilometres, though they often land very close to the take-off point.Balloon rallies may consist of just a few balloons for a one-day outing or up to several hundred balloons for a weeklong festival. Competitive events include distance within a time limit, spot landing, and “hare and hound” races. Hare and hound races are easy to organize and judge since they only require one (hare) balloon to launch first and fly a reasonable distance. The competitors attempt to land as close as possible to the hare's landing position. In crowded conditions, markers are often dropped to simulate the landings, and the balloons fly on to more open locations.
Commercial ride operators are in business almost everywhere in the world. Some ride balloons carry 10 to 20 passengers at a time in gigantic partitioned baskets. In California and France, wine-country flights are popular tourist attractions. African safari flights, at low altitude over vast game preserves, are perhaps the pinnacle of ride ballooning.
Hot-air balloon components
Envelope design
 Hot-air balloons vary considerably in design and materials. Lightweight coated-nylon and polyester fabrics are the most common materials for envelopes. Cotton is very serviceable but has a comparatively poor weight-to-strength ratio. Specially shaped balloons, which are literally pneumatic sculptures, are popular at public events. They utilize special tailoring and many internal baffles and cords to attain the desired designs.
Hot-air balloons vary considerably in design and materials. Lightweight coated-nylon and polyester fabrics are the most common materials for envelopes. Cotton is very serviceable but has a comparatively poor weight-to-strength ratio. Specially shaped balloons, which are literally pneumatic sculptures, are popular at public events. They utilize special tailoring and many internal baffles and cords to attain the desired designs. Sport balloons typically have a silhouette similar to the natural shape of fully inflated gas balloons. They can be assembled with many vertical gores (fabric sections, or panels) or fewer horizontal gores. The gore material can be cut straight (with the fabric's natural grain) or on the bias (diagonal to the fabric's natural grain). If straight gores are used, excess material can be gathered to create a fluted pattern that provides some flexibility. Gores made of bias-cut materials have greater stretch, which provides a natural flexibility. With horizontal gores, the individual panels can be gathered to provide a bulbous gore, which gives even greater flexibility. Because of the greatly reduced effective radius of curvature, a bulbous gore balloon experiences much less stress on the envelope fabric.
Sport balloons typically have a silhouette similar to the natural shape of fully inflated gas balloons. They can be assembled with many vertical gores (fabric sections, or panels) or fewer horizontal gores. The gore material can be cut straight (with the fabric's natural grain) or on the bias (diagonal to the fabric's natural grain). If straight gores are used, excess material can be gathered to create a fluted pattern that provides some flexibility. Gores made of bias-cut materials have greater stretch, which provides a natural flexibility. With horizontal gores, the individual panels can be gathered to provide a bulbous gore, which gives even greater flexibility. Because of the greatly reduced effective radius of curvature, a bulbous gore balloon experiences much less stress on the envelope fabric.With bias gores, load tapes known as tensors are generally sewn loosely into ducts formed in the vertical seams, much like the shroud lines sewn into a parachute's radial seams. With other balloons, the load is carried in tapes sewn or heat-sealed directly to the vertical seams. One load tape can and should have more than enough strength to carry the whole load without an excessive weight penalty. The stress in the skin of the balloon is so low that normal handling will cause visible damage if the fabric is weakened by wear or exposure long before it would fail in normal flight. The importance of load tapes and adequate excess strength in them in balloon construction cannot be overemphasized. Catastrophic failure in a properly designed balloon is extremely rare.
Deflation systems
Landing a bag with some four tons of air in it at 30 km (20 miles) per hour without wheels, steering gear, or brakes presents some problems. Prior to John Wise's discovery of the rip panel deflation system in the mid-19th century, a gas balloon could be dragged along for several kilometres before coming to a stop. In particular, balloons on extended flights seemed to be drawn into low pressure systems, which often resulted in stormy landings.
The basic hot-air balloon rip panel is a simple large sleeve at the apex that is drawn into a bunch and tied off with a cord. The cord is typically cut remotely by an electrically actuated explosive squib cannon or released with a pull cord moments before touchdown in order to deflate the envelope rapidly. This configuration is known as a pop top. Other balloon models use a circular panel held in place with a hook-and-loop (e.g., Velcro) closure that can be opened and closed progressively for finer adjustments in buoyancy. That system is more often replaced with Tracy Barnes's “ parachute top,” which is a combination venting and deflation panel. The parachute top consists of a simple hole at the apex of the balloon, usually about one-quarter of the balloon's diameter, plugged with a parachute of slightly greater diameter. The parachute is positioned by cords radiating out a few metres from the parachute's edge to anchors located on the puffy gore centres. A venting cord leads from the juncture of the parachute's shroud lines to the basket. Sometimes a mechanical advantage is gained by a pulley system. Pulling the cord draws the parachute down into the balloon, thereby letting hot air escape. Releasing the cord snaps the parachute back into the closed position with the force of the hot air. If the parachute is pulled far enough into the balloon, it will collapse, letting the balloon completely deflate rapidly. This is a great advantage in high-wind landings, where it might be difficult to maintain tension on the cord. Some versions use separate retracting cords to force the opening to close, redundant cinch cords to bunch the parachute for hands-off deflation, and elastic centring cords to provide automatic rapid setting and resetting of the parachute.
Burners
Hot-air balloons today use liquid propane burners exclusively. The burner's vaporizing coils are generally made of steel, but copper tubing has been used with good results. A secondary system without vaporization and slower air mixing can provide much quieter operation and give redundancy for safety. Many designs have used multiple duplicate burners and fuel supplies because there has been a history of leaking seals on control valves and occasionally contaminated fuel.
Baskets
Passenger baskets, or gondolas, vary as much in design as envelopes. For easy transportation, collapsible tubular frames with stout fabric covers can meet minimum requirements. Originally, wicker baskets had manila rope woven into the basket for suspension, but because of the potential for rot, these were replaced with synthetic or steel cables. Heavy wickerwork frames, using rattan up to a few centimetres in diameter, typically have also been replaced with metal or plastic framing.
Wicker construction has an advantage over metal skeletons and hard fibreglass shells in its absorption of the kinetic energy of impacts, however. Wicker is also favoured for its nostalgic artistic appearance. Any basket can have closed-cell foam padding on the inside for passenger safety and comfort.
Metallic components present hazards in the event of contact with electrical power lines. However, wickerwork by itself provides little protection to the passengers or fuel tanks from such contact. Vacuum-formed monocoque plastic baskets are slowly coming into use. They provide advanced impact and electrical contact resistance.
High-altitude ballooning
Beginning with the 18th century, ballooning has continually achieved higher altitudes. From Charles's 3,000-metre (10,000-foot) ascent in 1783 to U.S. Army Air Corps Capt. Hawthorne C. Gray's fatal ascent to 12,950 metres (42,470 feet) in 1927, the maximum altitude was only limited by the pilot's need for oxygen. Lacking confidence in the ability to seal an aircraft hermetically, American aviation pioneer Wiley Post (Post, Wiley) and others concentrated on individual pressure suits. Even as late as 1937, prominent aeronautical engineers publicly derided the concept of building airplane pressure cabins.
The Piccard invention of the stratosphere balloon (see above Balloons reach the stratosphere (balloon flight)) opened up new heights for exploration. The first stratosphere flights were mounted to study cosmic rays (cosmic ray), which are absorbed as they enter Earth's atmosphere. Early high-altitude work with plastic balloons continued cosmic ray research, air sampling for detecting atomic explosions, photographic flights over foreign terrain, astronomical observations above the disturbances of the troposphere, and even aerodynamic testing of free-falling payloads. A balloon is the only stable platform for any type of observation above the range of airplanes and below the range of orbital spacecraft. It is also the only aircraft that does not affect its environment and the only device that can sit relatively motionless above heights obtainable by helicopters.
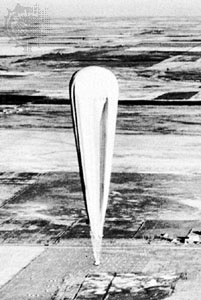 High-altitude plastic balloons are only partially inflated at launch to allow for gas expansion as the balloon climbs. This expansion is roughly tenfold for each 15,000 metres (50,000 feet) of altitude. When the balloon is at the designated altitude, the envelope takes on the natural shape—an inverted teardrop with the load attached at the bottom point. If the balloon were pressurized with no payload, the envelope would be spherical. There is a complete series of envelope shapes that depend on the ratio of payload-to-skin weight and internal pressure. Normally, the internal pressure is zero and the envelope weight is a high percentage of the total balloon weight. This gives a very fat balloon with an almost flat top. The included angle at the base can approach 45 degrees.
High-altitude plastic balloons are only partially inflated at launch to allow for gas expansion as the balloon climbs. This expansion is roughly tenfold for each 15,000 metres (50,000 feet) of altitude. When the balloon is at the designated altitude, the envelope takes on the natural shape—an inverted teardrop with the load attached at the bottom point. If the balloon were pressurized with no payload, the envelope would be spherical. There is a complete series of envelope shapes that depend on the ratio of payload-to-skin weight and internal pressure. Normally, the internal pressure is zero and the envelope weight is a high percentage of the total balloon weight. This gives a very fat balloon with an almost flat top. The included angle at the base can approach 45 degrees.Some heavy-payload high-altitude balloons have been made of nylon laminated to polyester film, but most research balloons use very thin polyethylene. Some of these polyethylene films are less than a tenth of a millimetre thick. In order to carry a payload, the seams are reinforced with load-bearing tape.
In flight the payload is generally suspended from an open extended parachute connected at its apex to the base of the balloon. The parachute can be released by radio control. At the moment of release, the elasticity of the parachute snaps it open almost instantly. A radio-controlled gas valve can be used at the top of the balloon. Excess gas is vented by ducts high on the side of the balloon, with their openings at the base level. If the duct is placed high enough on the balloon, it is impossible for outside air to enter the balloon.
Contamination of the lifting gas by outside air will lower the possible ceiling of the balloon and, in the case of hydrogen, create a fire hazard. The design must assure that flow resistance in the duct does not create adverse back pressure, which would burst the balloon. The diameter of the duct, its length, and any possible kinks must be considered. The weight of the duct itself can pull it down enough to block the opening into the balloon in some cases. With the duct design, any entrained air from mishandling during inflation or minute leaks in the balloon below the base of the lifting gas will pool in the base of the balloon and lower the ceiling accordingly.
The natural shape gives a balloon very low skin stress under static conditions. At altitude the balloon is very stable and not generally subject to turbulence or dynamic loads. On the way up, the balloon is only partially inflated and has great flexibility to distort, relieving any stress.
The first manned stratosphere balloon used a spherical aluminum cabin. Following ones used magnesium alloys and spun aluminum. Current high-altitude pressure cabins are made with various composite materials. Internal pressure is maintained by onboard liquid oxygen supplies and air scrubbers to remove carbon dioxide, moisture, and other body products.
Several flights have been made with no cabin at all. Crews in open gondolas wear space suits similar to those that astronauts wear.
Notable balloon altitude records Notable balloon altitude records
Long-distance ballooning
 Flying for ever greater distances has always been a goal of balloonists. The first successful aerial crossing of the English Channel occurred on Jan. 7, 1785, in a gas balloon piloted by French balloonist Jean-Pierre Blanchard and American balloonist John Jeffries. Another early long-distance flight was by the English balloonist Charles Green (Green, Charles), accompanied by the Irish musician Thomas (“Monck”) Mason, aboard the Great Balloon of Nassau in November 1836. Taking off from London, they traveled about 750 km (480 miles) in 18 hours to land in the Duchy of Nassau (now in Germany). Paul (“Ed”) Yost and Donald Piccard made the first hot-air balloon crossing of the English Channel in 1963.
Flying for ever greater distances has always been a goal of balloonists. The first successful aerial crossing of the English Channel occurred on Jan. 7, 1785, in a gas balloon piloted by French balloonist Jean-Pierre Blanchard and American balloonist John Jeffries. Another early long-distance flight was by the English balloonist Charles Green (Green, Charles), accompanied by the Irish musician Thomas (“Monck”) Mason, aboard the Great Balloon of Nassau in November 1836. Taking off from London, they traveled about 750 km (480 miles) in 18 hours to land in the Duchy of Nassau (now in Germany). Paul (“Ed”) Yost and Donald Piccard made the first hot-air balloon crossing of the English Channel in 1963.The New York Sun newspaper reported on April 13, 1844, that Monck Mason had made the first transatlantic balloon crossing, but the report turned out to be a hoax by Edgar Allan Poe (Poe, Edgar Allan). The actual first transatlantic balloon crossing occurred in 1978 aboard the Double Eagle II, a helium-filled balloon built by Yost, with piloting duties shared by three New Mexico businessmen, Ben L. Abruzzo (Abruzzo, Ben L.), Maxie Anderson (Anderson, Maxie), and Larry M. Newman. The first transpacific balloon flight was made in 1981 by Americans Abruzzo, Newman, Ron Clark, and Rocky Aoki aboard the helium-filled Double Eagle V.
In 1987 British entrepreneur Richard Branson (Branson, Sir Richard) and Swedish aeronaut Per Lindstrand, aboard the Virgin Atlantic Flyer, made the first transatlantic flight in a hot-air balloon. And in 1991, aboard the Otsuka Flyer, they made the first transpacific flight in a hot-air balloon. In 1984 American aviator Joseph W. Kittinger, aboard the helium-filled Rosie O'Grady's Balloon of Peace, made the first solo transatlantic balloon flight. In 1995 American adventurer Steve Fossett (Fossett, Steve), aboard the helium-filled Solo Challenger, made the first solo transpacific balloon flight.
Several around-the-world balloon flights were attempted with various systems, but success was finally achieved in 1999 by Swiss balloonist Bertrand Piccard (son of Jacques Piccard, grandson of Auguste Piccard, and second cousin of Donald Piccard) and British balloonist Brian Jones aboard a combination hot-air and helium balloon, the Breitling Orbiter III, with a pressurized cabin. The first solo around-the-world balloon flight was made by Fossett aboard a combination helium and hot-air balloon, the Bud Light Spirit of Freedom, in 2002.
The success of the Breitling depended on several independent factors. Balloon design, cabin design, and meteorological technique were all unique and individually critical. While it may be possible to use other techniques, all manned major long-distance efforts have utilized the jet stream. This limits the altitude, track, and season for a successful attempt to the winter months in mid-latitudes at elevations of about 6,000 to 10,000 metres (about 20,000 to 35,000 feet).
In order to navigate a balloon on a long-distance flight, the pilot must take advantage of meteorological conditions. Sensitive attention to altitude, rate of climb, and global positioning instrumentation is essential in order to follow minute-by-minute advice from ground-based weather coaches. Information, including complete weather maps, can be communicated by wireless Internet e-mail connections. For an intercontinental flight, which may take several days, reevaluation of computer-generated weather predictions is important; for global circumnavigation it is essential.
For a successful global voyage only a general meteorological condition can be chosen. It is impossible to calculate weather conditions two and three weeks in advance at locations around the world. The general condition and the immediate forecast govern the decision to launch. Once the balloon is airborne and on its way, the weather model must be constantly updated and the balloon navigated precisely to take advantage of varying conditions. While the aeronauts of the 19th century had balloons that could theoretically cross the Atlantic, all attempts failed because they lacked the meteorology to make accurate predictions and the means to communicate predictions to the balloonist.
Notable balloon flights Notable balloon flights
- box frame construction
- Boxhole Meteorite Crater
- boxing
- Boxing Day
- box lacrosse
- box set
- box turtle
- boxwood
- boxwork
- Boyacá
- Boyacá, Battle of
- boyar
- boy bishop
- Boyce, William
- Boy Charlton
- boycott
- Boycott, Charles Cunningham
- Boyd, Belle
- Boyd H. Bode
- Boyd, Martin
- Boyd-Orr of Brechin Mearns, John Boyd Orr, Baron
- Boyd, Robert Boyd, 1st Lord
- Boyd, William
- Boye, Karin
- Boyer, Charles