carbon cycle
ecology

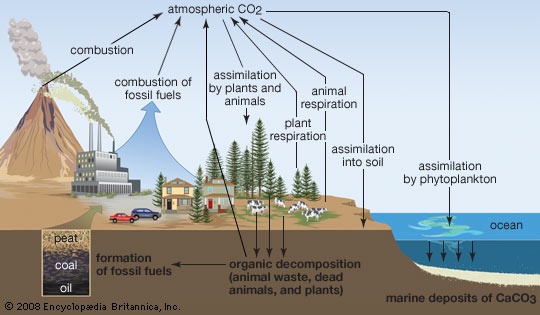 in biology, circulation of carbon in various forms through nature. Carbon is a constituent of all organic compounds, many of which are essential to life on Earth. The source of the carbon found in living matter is carbon dioxide (CO2) in the air or dissolved in water. Algae and terrestrial green plants (producers) are the chief agents of carbon dioxide fixation through the process of photosynthesis, through which carbon dioxide and water are converted into simple carbohydrates. These compounds are used by the producers to carry on metabolism, the excess being stored as fats and polysaccharides. The stored products are then eaten by consumer animals, from protozoans to man, which convert them into other forms. All animals return CO2 directly to the atmosphere as a by-product of their respiration. The carbon present in animal wastes and in the bodies of all organisms is released as CO2 by decay, or decomposer, organisms (chiefly bacteria and fungi) in a series of microbial transformations.
in biology, circulation of carbon in various forms through nature. Carbon is a constituent of all organic compounds, many of which are essential to life on Earth. The source of the carbon found in living matter is carbon dioxide (CO2) in the air or dissolved in water. Algae and terrestrial green plants (producers) are the chief agents of carbon dioxide fixation through the process of photosynthesis, through which carbon dioxide and water are converted into simple carbohydrates. These compounds are used by the producers to carry on metabolism, the excess being stored as fats and polysaccharides. The stored products are then eaten by consumer animals, from protozoans to man, which convert them into other forms. All animals return CO2 directly to the atmosphere as a by-product of their respiration. The carbon present in animal wastes and in the bodies of all organisms is released as CO2 by decay, or decomposer, organisms (chiefly bacteria and fungi) in a series of microbial transformations.Part of the organic carbon—the remains of organisms—has accumulated in the Earth's crust as fossil fuels (fossil fuel) (e.g., coal, gas, and petroleum), limestone, and coral. The carbon of fossil fuels, removed from the cycle in prehistoric time, is now being released in vast amounts as CO2 through industrial and agricultural processes, much of it quickly passing into the oceans and there being “fixed” as carbonates. If oxygen is scarce (as in sewage, marshes, and swamps) some carbon is released as methane gas.
nuclear fusion
also called carbon–nitrogen cycle
sequence of thermonuclear reactions that provides most of the energy radiated by the hotter stars. It is only a minor source of energy for the Sun and does not operate at all in very cool stars. Four hydrogen nuclei are in effect converted into one helium nucleus, a fraction of the mass being released as energy (according to the law of mass–energy equivalence, E = mc2). The German-U.S. physicist Hans Bethe (Bethe, Hans), in 1938, first described the process.
The reactions are as follows: a carbon-12 (12C) nucleus captures a hydrogen nucleus (1H, a proton) to form a nucleus of nitrogen-13 (13N); a gamma ray (γ) is emitted in the process. The nitrogen-13 nucleus emits a positive electron (positron, e+) and becomes carbon-13 (13C). This nucleus captures another proton, becomes nitrogen-14 (14N), and emits another gamma ray. The nitrogen-14 captures a proton to form oxygen-15 (15O); the resulting nucleus ejects a positron as above and is thereby transformed to nitrogen-15 (15N). Eventually, the nitrogen-15 nucleus captures a fast-moving proton and breaks down into a carbon-12 nucleus plus a helium nucleus (alpha particle) of mass 4 (4He).
In symbols:

- Hebrew
- Hebrew alphabet
- Hebrew language
- Hebrew literature
- Hebrews, Letter to the
- Hebrew Union College
- Hebrew University of Jerusalem
- Hebrides
- Hebron
- Heb-Sed
- Hecataeus of Miletus
- Hecate
- Hecate Strait
- Hecatompylos
- Hechingen
- Hecht, Anthony
- Hecht, Ben
- Hechtia
- Hechuan
- Heckel, Erich
- heckelphone
- Hecker, Friedrich
- Hecker, Isaac Thomas
- Hecker, Johann Julius
- Heckman, James J.