cluster
chemistry and physics
Introduction
Atoms and molecules are the smallest forms of matter typically encountered under normal conditions and are in that sense the basic building blocks of the material world. There are phenomena, such as lightning and electric discharges of other kinds, that allow free electrons to be observed, but these are exceptional occurrences. It is of course in its gaseous state that matter is encountered at its atomic or molecular level; in gases each molecule is an independent entity, only occasionally and briefly colliding with another molecule or with a confining wall.
In contrast to the free-molecule character of gases, the condensed phases of matter—as liquids, crystalline solids, and glasses are called—depend for their properties on the constant proximity of all their constituent atoms. The extent to which the identities of the molecular constituents are maintained varies widely in these condensed forms of matter. Weakly bound solids, such as solid carbon dioxide ( Dry Ice), or their liquid counterparts, such as liquid air or liquid nitrogen, are made up of molecules whose properties differ only slightly from the properties of the same molecules in gaseous form; such solids or liquids are simply molecules packed tightly enough to be in constant contact. These are called van der Waals (van der Waals forces) solids or liquids, after Johannes D. van der Waals, the Dutch physicist who described the weak forces that just manage to hold these materials together if they are cold enough. In other solids, like diamond, graphite, silicon, or quartz, the individual atoms retain their identity, but there are no identifiable molecules in their structures. The forces between the constituent atoms are roughly as strong as the forces that hold atoms together in the strongly bound covalent molecules that make up most common substances. Negatively charged electrons act as a “glue” to hold the positively charged nuclei together and are more or less confined to the vicinity of the so-called home-base nuclei with which they are associated; they are not free to roam through the entire solid or liquid. These materials are said to be covalently bound and are electrical insulators. They are best described as neutral atoms held together by covalent bonds and are essentially one giant molecule.
Another kind of bonding found in condensed matter is exhibited by sodium chloride, ordinary table salt, which is composed of positive sodium ions (Na+) and negative chloride ions (Cl-). Such ionic (ionic bond) compounds are held together by the mutual attraction of the oppositely charged ions; because of their locations, these attractions are stronger than the repulsions of the ions with like charges. Each ion in an ionic crystal is surrounded by nearest neighbours of opposite charge. The consequence is that the binding energies of ionic compounds are large, comparable to those of strongly bound covalent substances.
Metallic bonding (metallic bond) is another type of binding found in condensed matter. Electrons moving between the positive atomic cores (i.e., the nuclei plus inner-shell, tightly bound electrons) form an electron cloud; the attractions between the positive cores and the negative charges that make up the cloud hold metals together. Metals differ from covalently bound insulators in that those electrons responsible for the cohesion of the metals move freely throughout the metal when given the slightest extra energy. For example, under the influence of the electric field produced in a copper wire when its ends are connected to the terminals of a battery, electrons move through the wire from the end connected to the battery's negative pole toward the end connected to its positive pole. An electric field applied to a metal generates an electric current, but the same electric field applied to a covalent insulator does not (see below Comparison with other forms of matter (cluster)). The net binding forces between electrons and atomic cores of a metal are comparable in strength to those that hold ionic compounds together.
As mentioned above, liquids constitute a condensed or dense phase of matter, but their atomic arrangement differs from that of solids. In a liquid the constituent atoms are only slightly farther apart than they are in a solid, but that small difference is significant enough to allow the atoms or molecules that constitute a liquid to move around and to assume a full range of geometric configurations. Atoms of the same kind can trade places and can wander through the liquid by the random-walk process called diffusion. In general, materials that can form solids can also form liquids, but some, such as carbon dioxide, can only enter the liquid state under excess pressure. At least one substance, helium, can form a liquid while having no known solid form.
Materials that form solids and liquids can exhibit another form, one that may be solidlike or liquidlike but that has properties somewhat different from those of the bulk. This is the form of matter consisting of exceedingly small particles that are called clusters. Clusters are aggregates of atoms, molecules, or ions that adhere together under forces like those that bind the atoms, ions, or molecules of bulk matter; because of the manner in which they are prepared, clusters remain as tiny particles at least during the course of an experiment. There are clusters held together by van der Waals forces, by ionic forces, by covalent bonds, and by metallic bonds. Despite the similarity of the forces that bind both clusters and bulk matter, one of the fascinating aspects of clusters is that their properties differ from those of the corresponding bulk material; that characteristic affords the opportunity to learn about the properties of bulk matter by studying how, as the number of constituent particles increases, the properties of clusters evolve into those of bulk matter. For example, a cluster of 20 or 30 atoms typically has a melting point far lower than that of the corresponding bulk. The electrical properties of clusters also differ in some instances from those of the bulk matter: clusters of only a few atoms of mercury are insulators, held together by weak van der Waals forces, but clusters of hundreds of mercury atoms are metallic. One of the puzzles posed by clusters is the question of how properties of small clusters evolve with size into properties of bulk matter.
Comparison with other forms of matter
Comparison with bulk matter
Several characteristics differentiate clusters from molecules and bulk matter. They differ from bulk matter, first and foremost, in size; whether three particles bound together constitute a cluster is a matter of choice and convention, but an aggregate of four or more atoms or molecules certainly comprises a cluster. Such a small cluster would differ markedly from bulk matter in almost all its properties. A second difference between clusters and bulk matter is the variability of the properties of clusters with the number of their constituent particles. The properties of a lump of bulk matter remain unchanged by the addition or subtraction of a few atoms or molecules, whereas the properties of a small cluster vary significantly and, in general, neither uniformly nor even in the same direction with a change in the number of constituent particles. Medium-size clusters have properties that vary smoothly with the number of constituent particles (denoted N), but their properties, such as the melting point, differ significantly from those of the corresponding bulk. Large clusters have properties that vary smoothly with N and clearly merge into those of their bulk counterparts. This distinction, while not extremely precise, is quite useful. For example, the average binding energies (binding energy)—that is, the average energy per constituent atom or molecule required to separate the particles from each other—vary widely with N for small clusters. The reason for this wide range is that clusters of certain values of N, known as magic numbers, can take on unusually stable geometric structures that yield large binding energies, while others with different small values of N have no especially stable forms and therefore only relatively low binding energies. The binding energies of medium-size clusters vary rather smoothly with N, but they are in general considerably lower than the binding energies of bulk matter. The most important reason for this trend is that in a body of bulk matter almost all the particles are in the interior, while in a cluster most of the particles are on the surface. In a cluster of 13 atoms of copper or argon, for example, 12 of the atoms are on the surface. In a cluster of 55 argon atoms, 42 atoms are on the surface, and, in a cluster of 137 argon atoms, 82 are on the surface. Surface atoms are bonded only to atoms in their own layer and to those directly beneath them, so they have fewer atoms holding them to the main body of matter, whether cluster or bulk, than do atoms in the interior. Hence, the average binding energies of atoms in clusters are normally considerably less than those of bulk matter.
An important difference between clusters, in particular small and medium-size clusters, and bulk solids is the structure that is assumed by their most stable form. Most bulk solids are crystalline (crystal). This means that their atomic structures consist of periodic lattices—i.e., structures that repeat over and over so that every unit composed of a few neighbouring atoms is indistinguishable from other groups of atoms that have exactly the same arrangement. In a simple cubic crystal, for example, all the atoms lie at the corners of cubes (in fact at a point common to eight equivalent cubes), and all these lattice points are identical. Such structures are called periodic. Most clusters, by contrast, have structures that are not periodic; many have the form of icosahedrons, incomplete icosahedrons, or other polyhedral structures that cannot grow into periodic lattices. One of the challenging puzzles of cluster science is to explain how, as an aggregate grows, it transforms from a polyhedral cluster-type structure into a crystalline lattice-type structure.
Furthermore, some properties of clusters reflect their small size in more subtle ways that depend on quantum (quantum mechanics) mechanical phenomena. These are generally much more pronounced in exceedingly small systems than in bulk or macroscopic samples. One such property is the nature of the energy levels (energy state) occupied by the electrons (electron). In a macroscopic sample the energies of the states available to an electron are, in principle, discrete but are merged into bands consisting of many energy levels. Within each band the intervals of energy between those discrete levels are too tiny to be discerned; only the gaps between the bands are large enough to be important because they correspond to ranges of energy that are forbidden to the electrons. In fact, it is the contrast in the mobility of electrons that differentiates insulators (insulator) from electrical conductors. In even a very cold metal, only an infinitesimal amount of excess energy is required to promote a few electrons into the previously empty energy levels in which they can move freely throughout the material. If an electric field is applied to the metal, the negatively charged electrons move toward the positive pole of the field so that a net current flows in the metal. It is the motion of these electrons, driven by an applied field, that makes metals conductors of electricity. In an insulator the electrons fill all the energy levels up to the top of the highest-energy occupied band. This means that at least the full energy of the forbidden interval, called the band gap, must be imparted to any electron to promote it to an allowed state where it may travel readily through the material. In an insulator this is far more energy than is normally available, and so no electrons are in states that allow them to move freely; such materials cannot conduct electric currents.
Clusters containing only a small number of metal atoms have so few available quantum states for their electrons that these states must be considered discrete, not as components of a dense band of available states. In this sense, small clusters of metal atoms are like conventional molecules rather than like bulk metals. Medium-size clusters of metal atoms have electronic energy states that are close enough together to be treated like the bands of bulk metals, but the conducting properties of these clusters are different from those of the bulk. Electrons driven by a constant electric field in a bulk metal can travel distances that are extremely long compared with atomic dimensions before they encounter any boundaries at the edges of the metal. Electrons in metal clusters encounter the boundaries of their cluster in a much shorter distance. Hence, metal clusters do not conduct electricity like bulk metals; if they are subjected to rapidly oscillating electric fields, such as those of visible, infrared, or microwave radiation, their “free” electrons are driven first one way and then back in the opposite direction over distances smaller than the dimensions of the cluster (see below Physical properties (cluster)). If they are subjected to constant or low-frequency electric fields, such as the common 60-hertz fields that drive ordinary household currents, the electrons reach the boundaries of their clusters and can go no farther. Thus, the equivalent of conduction is not seen at low frequencies.
Comparison with molecules (molecule)
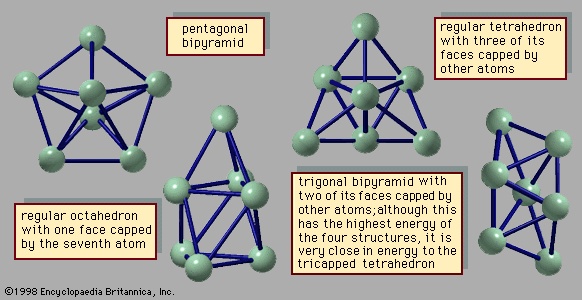
 The manner in which clusters differ from molecules is more of a categorical nature than one of physical properties. Molecules have a definite composition and geometry; with few exceptions clusters can be made of any number of particles and may have any of several geometries. The four possible structures of a cluster of seven argon atoms are shown in Figure 1-->
The manner in which clusters differ from molecules is more of a categorical nature than one of physical properties. Molecules have a definite composition and geometry; with few exceptions clusters can be made of any number of particles and may have any of several geometries. The four possible structures of a cluster of seven argon atoms are shown in Figure 1--> , and the lowest and next three higher-energy structures of a 13-atom cluster of argon are illustrated in Figure 2-->
, and the lowest and next three higher-energy structures of a 13-atom cluster of argon are illustrated in Figure 2--> . The 13-atom cluster has the form of a regular icosahedron of 12 argon atoms around a central atom and is particularly stable.
. The 13-atom cluster has the form of a regular icosahedron of 12 argon atoms around a central atom and is particularly stable.Despite their multiplicity of structures, small clusters of fixed size, undergoing vibrations of small amplitude around a single geometry, are in most respects indistinguishable from molecules. If such clusters are given energy that is not great enough in magnitude to break them into separate parts, they may assume other geometries, alternating among these structural forms. This phenomenon is rarely seen with conventional molecules, but it is not unknown for energized molecules to exhibit more than one structure and to pass among them.
All in all, small clusters are much like molecules and are often considered to be molecules, while very large clusters are quite similar to bulk matter. The properties of clusters whose size is between these extremes may be like either or like neither.
Methods of study
Clusters can be studied by experiment, by theoretical analysis, and by simulation with computer-generated models. For several reasons they cannot be studied in the same manner as bulk matter. First, if individual clusters are allowed to coalesce into a mass, they will actually turn into bulk matter, so they must be kept separated. Second, it is desirable (but not always possible) to conduct experiments that distinguish the size and structure of each kind of cluster under observation. Because of these two considerations, experiments with clusters are usually more difficult than those with either specific molecules or bulk matter. Most of the difficulties arise from the same properties that make clusters interesting: the ease with which their sizes and compositions are varied and the variety of structures available for clusters of almost any given size.
Preparation of clusters
Because of these difficulties, most experiments on clusters have been carried out with the clusters isolated in the gas phase; a few studies have been done with them in solution or in frozen matrices. Clusters can be prepared in the gas phase and then either studied in that form or captured into solvents or matrices or onto surfaces. They may be made by condensation of atoms or molecules or by direct blasting of matter from solids. In the most generally used method, a gas containing the gaseous cluster material is cooled by passing it under high pressure through a fine hole or slot. The expansion cools the gas rapidly from its initial temperature—usually room temperature but much higher if the cluster material is solid at room temperature—to a temperature not far above absolute zero. If, for example, argon gas is expanded in this way, it condenses into clusters if the pressure is not too high and the aperture is not too small; if the conditions are too extreme, the argon instead turns to snow and condenses.
Inert gases (noble gas) are often used as the medium by which other materials, in a gaseous or vaporous state, are transported from the ovens or other sources where they have been gasified and through the jets that cool them and turn them into clusters. One especially popular and interesting method in which solids are vaporized (vaporization) is by the action of intense laser beams on solid surfaces. Often called ablation, this process is an effective means of vaporizing even highly refractory materials like solid carbon. The ablated material is then carried through the cooling jet by an inert gas such as helium or argon.
ionization and sorting of clusters
Once the clusters have been formed, they can be studied in a variety of ways. One of the first techniques was simply to ionize the clusters, either with ultraviolet radiation (usually from a laser) or by electron impact. The gaseous ionized clusters are accelerated by an electric field and then analyzed according to their masses (see mass spectrometry); these results immediately reveal the number of atoms or molecules in the cluster. The analysis yields the distribution of the relative abundances of clusters of different sizes in the beam. If the experiment is done with considerable care, the abundance distribution corresponds to the true relative stabilities of the clusters of different sizes. However, like many experiments with clusters, these can either provide results consistent with the equilibrium conditions that reflect those relative stabilities, or they can give results that reflect the rates of the cluster-forming processes rather than the equilibrium characteristics, as the latter may take far longer to reach than the time required to form clusters. Some of the implications of the abundances found in such experiments are discussed below in the section Structure and properties: Structure.
Because of the conditions under which clusters are formed, their distributions contain many different sizes and, in some instances, different shapes. Because chemists seek to characterize clusters of a single size and geometry, the clusters must first be sorted on that basis. If the clusters carry charge, they can be separated according to size with a mass spectrometer that sorts charged particles with approximately the same energy according to their masses. This is usually done by deflecting the charged clusters or ions with an electric or magnetic field; the smaller the mass, the greater is the deflection. This is one of the most effective ways of preparing a beam of clusters of only a single selected mass. It does not eliminate the problem of multiple structures, however.
A technique that can sometimes be used to sort clusters according to their size and structure is a two-step process in which one cluster species at a time is excited with the light from a laser and is then ionized with light from a second laser. This process, called resonant two-photon ionization, is highly selective if the clusters being separated have moderately different absorption spectra. Since this is frequently the case, the method is quite powerful. As the experimenter varies the wavelength of the first exciting laser, a spectrum is produced that includes those wavelengths of light that excite the cluster. If the wavelength of the second ionizing laser is varied, the method also yields the ionization potential, which is the minimum energy that the photon in the ionizing beam must possess in order to knock an electron out of the cluster. Such data help to reveal the forces that bind the cluster together and give some indication of how the cluster will react with atoms, molecules, or other clusters.
computer simulation of cluster behaviour
A powerful tool for studying clusters is computer simulation of their behaviour. If the nature of the forces between the individual atoms or molecules in a cluster is known, then one can construct a computer model that represents the behaviour of those atoms or molecules by solving the equations of motion (motion, equation of) of the cluster. To describe the cluster in terms of classical mechanics, the Newtonian equations of motion are solved repeatedly—namely, force equals mass times acceleration, in which the forces depend on the instantaneous positions of all the particles. Hence, these equations are simultaneous, interlinked equations; there is one set of three (for the three instantaneous coordinates of each particle) for each atom or molecule. The results can take one of three forms: (1) the positions and coordinates of the atoms, given in tables, (2) the average properties of the entire cluster, or (3) animations. Tables are too cumbersome for most purposes, and specific average properties are frequently what the investigator seeks. Animated sequences show the same content as the tables but far more efficiently than extensive tables do. In fact, animations sometimes reveal considerably more than is expected by scientists.
It is also possible to construct computer models of clusters based on quantum mechanics instead of Newton's classical mechanics. This is especially appropriate for clusters of hydrogen and helium, because the small masses of their constituent atoms make them very quantumlike in the sense that they reveal the wavelike character that all matter exhibits according to quantum mechanics. The same kinds of data and inferences can be extracted from quantum mechanical calculations as from classical ones, but the preparation and visualization of animations for such clusters are much more demanding than their classical mechanical counterparts.
Structure and properties
Structure
The abundance distributions for several kinds of clusters show that there are certain sizes of clusters with exceptional stability, analogous to the exceptional stability of the atoms of the inert gases helium, neon, argon, krypton, and xenon and of the so-called magic number nuclei—i.e., the sequence of unusually stable atomic nuclei beginning with the α-particle, or helium nucleus. Such unusual stability suggests that its interpretation should be associated with the closing of some kind of shell, or energy level. The overall structure that determines the cluster's stability is generally called its shell structure.
Clusters with icosahedral structures
 Clusters of atoms bound by van der Waals forces or by other simple forces that depend only on the distance between each pair of atoms have unusual stability when the cluster has exactly the number of atoms needed to form a regular icosahedron. The first three clusters in this series have, respectively, 13, 55, and 147 atoms. These are shown in Figure 3-->
Clusters of atoms bound by van der Waals forces or by other simple forces that depend only on the distance between each pair of atoms have unusual stability when the cluster has exactly the number of atoms needed to form a regular icosahedron. The first three clusters in this series have, respectively, 13, 55, and 147 atoms. These are shown in Figure 3--> . In the 13-atom cluster, all but one of the atoms occupy equivalent sites. The 55-atom cluster in this series consists of a core—which is just the 13-atom icosahedron—plus 12 more atoms atop the 12 vertices of the icosahedron and 30 more atoms, one in the centre of each of the 30 edges of the icosahedron. The 147-atom cluster consists of a 55-atom icosahedral core, 12 more atoms at the vertices of the outermost shell, one atom in the centre of each of the 20 faces, and two atoms along each of the 30 edges between the vertices. The shell structure that provides special stabilities in this class of clusters is determined by the individual stabilities of the shells of the atoms themselves.
. In the 13-atom cluster, all but one of the atoms occupy equivalent sites. The 55-atom cluster in this series consists of a core—which is just the 13-atom icosahedron—plus 12 more atoms atop the 12 vertices of the icosahedron and 30 more atoms, one in the centre of each of the 30 edges of the icosahedron. The 147-atom cluster consists of a 55-atom icosahedral core, 12 more atoms at the vertices of the outermost shell, one atom in the centre of each of the 20 faces, and two atoms along each of the 30 edges between the vertices. The shell structure that provides special stabilities in this class of clusters is determined by the individual stabilities of the shells of the atoms themselves.Clusters of simple metal atoms
A different kind of extraordinary stability manifests itself in clusters of simple metal atoms. The shell structure for this class of clusters is determined by the electrons (electron) and the filling of those shells that have energy states available to the electrons. The numbers of electrons corresponding to closed electron shells in metal clusters are 8, 20, 40, 58, . . . . The electron structure can be modeled by supposing that the positively charged cores consisting of the protons and inner-shell electrons of all the cluster's atoms are smeared out into a continuous, attractive background, while the valence, or outer-shell, electrons are delocalized (i.e., shared among all atoms in the cluster). The electron environment is much like a well or pit with a flat bottom and a moderately steep wall. The determination of the energy states available for electrons in such a simplified model system is relatively easy and gives a good description of clusters of more than about eight or nine alkali (alkali metal) atoms—i.e., lithium, sodium, potassium, rubidium, or cesium. The single valence, or outer-shell, electron of each alkali atom is treated explicitly, while all the others are considered part of the smeared-out core. Since each alkali atom has only one valence electron, the unusually stable clusters of alkalis consist of 8, 20, 40, . . . atoms, corresponding to major shell closings. This model is not as successful in treating metals such as aluminum, which have more than one valence electron.
Network structures
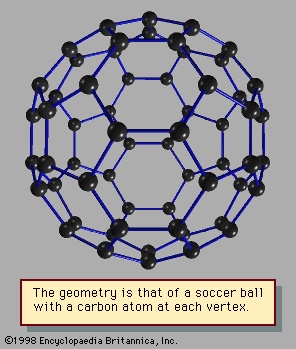 Still another kind of particularly stable closed shell occurs in clusters sometimes called network structures. The best-known of these is 60, the 60-atom cluster of carbon atoms. In this cluster the atoms occupy the sites of the 60 equivalent vertices of the soccer ball structure, which can be constructed by cutting off the 12 vertices of the icosahedron to make 12 regular 5-sided (regular pentagonal) faces. The icosahedron itself has 20 triangular faces; when its vertices are sliced off, the triangles become hexagons. The 12 pentagons share their edges with these 20 hexagonal faces. No two pentagons have any common edge in this molecule or cluster (C60 may be considered either). The resulting high-symmetry structure has been named buckminsterfullerene, after R. Buckminster Fuller (Fuller, R Buckminster), who advocated using such geometric structures in architectural design (see Figure 4-->
Still another kind of particularly stable closed shell occurs in clusters sometimes called network structures. The best-known of these is 60, the 60-atom cluster of carbon atoms. In this cluster the atoms occupy the sites of the 60 equivalent vertices of the soccer ball structure, which can be constructed by cutting off the 12 vertices of the icosahedron to make 12 regular 5-sided (regular pentagonal) faces. The icosahedron itself has 20 triangular faces; when its vertices are sliced off, the triangles become hexagons. The 12 pentagons share their edges with these 20 hexagonal faces. No two pentagons have any common edge in this molecule or cluster (C60 may be considered either). The resulting high-symmetry structure has been named buckminsterfullerene, after R. Buckminster Fuller (Fuller, R Buckminster), who advocated using such geometric structures in architectural design (see Figure 4--> ).
).Other network compounds of carbon are also known. To form a closed-shell structure, a network compound of carbon must have exactly 12 rings of 5 carbon atoms, but the number of rings of 6 carbon atoms is variable. Shells smaller than C60 have been discovered, but some of their constituent pentagons must share edges; this makes the smaller network compounds less stable than C60. Shells larger than C60, such as C70, C76, and C84, are known and are relatively stable. Even tubes and “onions” of concentric layers of carbon shells have been reported in observations made with modern electron microscopes known as scanning tunneling microscopes. These devices are powerful enough to reveal images of extremely small clusters and even individual foreign atoms deposited on clean surfaces.
The network compounds of carbon, which make up the class called fullerenes (fullerene), form compounds with alkali and other metals. Some of these compounds of fullerenes combined with metals, such as K3C60, become superconductors (superconductivity) at low temperatures; that is to say, they lose all resistance to electric current flow when they are cooled sufficiently. The class of network compounds as a group had been imagined from time to time, but only in the late 1980s were they realized in the laboratory and shown to have closed-shell network structures.
Physical properties
Liquid and solid phases
Clusters share some of the physical properties of bulk matter, a few of which are rather surprising. Clusters of all substances except helium and possibly hydrogen are solidlike at low temperatures as expected. The atoms or molecules of a cluster remain close to their equilibrium positions, vibrating around these positions in moderately regular motions of small amplitude. This is characteristic of all solids (solid); their atoms are constrained to stay roughly in the same position at all times. In a liquid or a gas, the atoms or molecules are free to wander through the space accessible to the substance. A gas or vapour has so much empty space relative to the volume occupied by the particles that the particles move almost unhindered, colliding only occasionally with other particles or with the walls of the container. A liquid is typically almost as dense as a solid but has some empty spaces into which the atoms or molecules can easily move. Hence, the particles of a liquid can diffuse with moderate ease. ( water is an exception; its density as a liquid is higher than its density as ice, because ice has an unusually open structure in comparison with most solids, and this open structure collapses when ice turns to water.) Clusters can be liquidlike if they are warm enough, but typically the temperatures at which clusters can become liquid are much lower than the melting points of the corresponding bulk solids. If temperatures are measured on the Kelvin scale, small clusters become liquidlike at temperatures of roughly half the bulk melting temperatures. For example, solid argon melts at approximately 80 K, while small clusters of argon become liquid at about 40 K.
Some clusters are expected to show a gradual transition from solidlike to liquidlike, appearing slushy in the temperature range between their solidlike and liquidlike zones. Other clusters are expected to show, as seen in computer simulations, distinct solidlike and liquidlike forms that qualitatively resemble bulk solids and liquids in virtually every aspect, even though they may exhibit quantitative differences from the bulk. Solid clusters, for example, show virtually no diffusion, but the particles of a liquid cluster can and do diffuse. The forces that hold a particle in place in a solid cluster are strong, comparable to those of a bulk solid; but those in a liquid cluster include, in addition to forces comparable in strength to those in solids, some forces weak enough to allow a particle to wander far from its home base and find new equilibrium positions. Those same weak forces are responsible for making a liquid cluster compliant; that is, weak forces allow the liquid to accommodate (cohesion) any new force, say, a finger inserted into water. Ice will not yield to such an intruding force, but when a finger is placed into liquid water, the water molecules move aside under the force of the finger. This is much like the behaviour of a bulk liquid. The greatest differences between bulk solids and liquids and solid and liquid clusters arise from the fact that a large fraction of the particles of a cluster are on its surface. As a result, the particle mobility that characterizes liquids and enables them to exhibit diffusion and physical compliance is enhanced in a cluster, for the cluster can easily expand by enlarging the spaces between particles and can also transfer particles from its interior to its surface, leaving vacancies that enhance the mobility of the interior particles. The large surface area, together with the curved shape of the cluster's surface, make it easier for particles to leave a cluster than to leave the flat surface of a bulk liquid or solid. An important consequence is that the vapour pressure of a cluster is higher than the vapour pressure of the corresponding bulk, and accordingly the boiling point of a liquid cluster—i.e., the temperature at which the vapour pressure of a liquid is equal to the pressure of the surrounding atmosphere—is lower than that of the corresponding bulk liquid. The vapour pressure of clusters decreases with increasing cluster size, while the boiling point increases.
Perhaps the greatest difference between clusters and bulk matter with regard to their transformation between solid and liquid is the nature of the equilibrium between two phases. Bulk solids can be in equilibrium with their liquid forms at only a single temperature for any given pressure or at only a single pressure for any given temperature. A graph of the temperatures and pressures along which the solid and liquid forms of any given substance are in equilibrium is called a coexistence curve (phase diagram). One point on the coexistence curve for ice and liquid water is 0° C and one atmosphere of pressure. A similar curve can be drawn for the coexistence of any two bulk phases, such as liquid and vapour; a point on the coexistence curve for liquid water and steam is 100° C and one atmosphere of pressure. Clusters differ sharply from bulk matter in that solid and liquid clusters of the same composition are capable of coexisting within a band of temperatures and pressures. At any chosen pressure, the proportion of liquid clusters to solid clusters increases with temperature. At low temperatures the clusters are solid, as described above. As the temperature is increased, some clusters transform from solid to liquid. If the temperature is raised further, the proportion of liquid clusters increases, passing through 50 percent, so that the mixture becomes predominantly liquid clusters. At sufficiently high temperatures all the clusters are liquid.
No cluster remains solid or liquid all the time; liquidlike clusters occasionally transform spontaneously into solidlike clusters and vice versa. The fraction of time that a particular cluster spends as a liquid is precisely the same as the fraction of clusters of that same type within a large collection that are liquid at a given instant. That is to say, the time average behaviour gives the same result as the ensemble average, which is the average over a large collection of identical objects. This equivalence is not limited to clusters; it is the well-known ergodic property that is expected of all but the simplest real systems.
Electric (electricity), magnetic, and optical properties
Other significant physical properties of clusters are their electric, magnetic, and optical properties. The electric properties of clusters, such as their conductivity and metallic or insulating character, depend on the substance and the size of the cluster. Quantum theory attributes wavelike character to matter, a behaviour that is detectable only when matter is examined on the scale of atoms and electrons. At a scale of millimetres or even millionths of millimetres, the wavelengths of matter are too short to be observed. Clusters are often much smaller than that, with the important consequence that many are so small that when examined their electrons and electronic states can exhibit the wavelike properties of matter. In fact, quantum properties may play an important role in determining the electrical character of the cluster. In particular, as described previously, if a cluster is extremely small, the energy levels or quantum states of its electrons are not close enough together to permit the cluster to conduct electricity.
Moreover, an alternative way to view this situation is to recognize that a constant electric force (i.e., the kind that drives a direct current) and an alternating force (the kind that generates alternating current) can behave quite differently in a cluster. Direct current cannot flow in an isolated cluster and probably cannot occur in a small cluster even if it is sandwiched between slabs of metal. The current flow is prohibited both because the electrons that carry the current encounter the boundaries of the cluster and because there are no quantum states readily available at energies just above those of the occupied states, which are the states that must be achieved to allow the electrons to move. However, if a field of alternating electric force is applied with a frequency of alternation so high that the electrons are made to reverse their paths before they encounter the boundaries of the cluster, then the equivalent of conduction will take place. Ordinary 60-cycle (60-hertz) alternating voltage and even alternations at radio-wave frequencies switch direction far too slowly to produce this behaviour in clusters; microwave frequencies are required.
Magnetic (magnetism) properties of clusters, in contrast, appear to be rather similar to those of bulk matter. They are not identical, because clusters contain only small numbers of electrons, which are the particles whose magnetic character makes clusters and bulk matter magnetic. As a result, the differences between magnetic properties of clusters and of bulk matter are more a matter of degree than of kind. Clusters of substances magnetic in the bulk also tend to be magnetic. The larger the cluster, the more nearly will the magnetic character per atom approach that of the bulk. The degree of this magnetic character depends on how strongly the individual electron magnets couple to each other to become aligned in the same direction; the larger the cluster, the stronger is this coupling.
The optical properties of weakly bound clusters are much like those of their component atoms or molecules; the small differences are frequently useful diagnostics of how the cluster is bound and what its structure may be. Optical properties of metal clusters are more like those of the corresponding bulk metals than like those of the constituent atoms. These properties reveal which cluster sizes are unusually stable and therefore correspond to “magic-number” sizes. Optical properties of covalently bound clusters are in most cases—e.g., fullerenes—unlike those of either the component atoms or the bulk but are important clues to the structure and bonding of the cluster.
Chemical properties
The chemical properties of clusters are a combination of the properties of bulk and molecular matter. Several kinds of clusters, particularly those of the metallic variety, induce certain molecules to dissociate (dissociation). For example, hydrogen molecules, H2, spontaneously break into two hydrogen atoms when they attach themselves to a cluster of iron atoms. ammonia likewise dissociates when attaching itself to an iron cluster. Similar reactions (reaction rate) occur with bulk matter, but the rate at which such gases react with bulk metals depends only on how much gaseous reactant is present and how much surface area the bulk metal presents to the gas. Metal powders react much faster than dense solids with the same total mass because they have much more surface area. Small and medium-size clusters, on the other hand, show different reactivities for every size, although these reactivities do not vary smoothly with size. Furthermore, there are instances, such as reactions of hydrogen with iron, in which two different geometric forms of clusters of a single size have different reaction rates, just as two different molecules with the same elemental composition, called chemical isomers (isomerism), may have different reaction rates with the same reactant partner. In the case of molecules, this is not surprising, because different isomers typically have quite different structures, physical properties, and reactivities and do not normally transform readily into one another. Isomers of clusters of a specific chemical composition, however, may well transform into one another with moderate ease and with no excessive increase in energy above the amount present when they formed. If the reaction releases energy (i.e., it is exothermic) in sufficient quantity to transform the cluster from solid to liquid, a cluster may melt as it reacts.
Some of the interesting chemistry of clusters is set in motion by light. For example, light of sufficiently short wavelength can dissociate molecules that are captured in the middle of a cluster of nonreactive atoms or molecules. A common question is whether the surrounding molecules or atoms form a cage strong enough to prevent the fragment atoms from flying apart and from leaving the cluster. The answer is that, if there are only a few surrounding atoms or molecules, the fragments escape their initial cage, and, if the energy of the light is high enough, at least one of the fragments escapes. On the other hand, if there are enough nonreacting cage atoms or molecules in the cluster to form at least one complete shell around the molecule that breaks up, the cage usually holds the fragments close together until they eventually recombine.
A related sort of reaction, another example of competition between a particle's attempt to escape from a cluster and some other process, occurs if light is used to detach an excess electron from a negative ion in the middle of an inert cluster. If, for example, light knocks the extra electron off a free, negatively charged bromine molecule, Br2-, the electron of course escapes. If the charged molecule is surrounded by a few inert molecules of carbon dioxide (CO2), the electron escapes almost as readily. If 10 or 15 CO2 molecules encase the Br2-, the electron does not escape; instead, it loses its energy to the surrounding molecules of CO2, some of which boil off, and then eventually recombines with the now neutral bromine molecule to re-form the original Br2-.
The chemical properties of fullerenes and other network compounds have become a subject of their own, bridging molecular and cluster chemistry. These compounds typically react with a specific number of other atoms or molecules to form new species with definite compositions and structures. Compounds such as K3C60 mentioned previously have the three potassium atoms outside the C60 cage, all as singly charged ions, K+, and the ball of 60 carbon atoms carries three negative charges to make the entire compound electrically neutral. Other compounds of C60, such as that made with the metal lanthanum, contain the metal inside the carbon cage, forming a new kind of substance. It is possible to add or take away hydrogen atoms from C60 and its larger relatives, much as hydrogen atoms can be added or removed from some kinds of hydrocarbons; in this way some of the chemistry of this class of clusters is similar to classical organic chemistry.
One of the goals of cluster science is the creation of new kinds of materials. The possible preparation of diamond films is one such application; another example is the proposal to make so-called superatoms that consist of an electron donor atom in the centre of a cluster of electron acceptors; the fullerene clusters containing a metal ion inside the cage seem to be just such a species but with much more open structures than had been previously envisioned. Molecular electronics (integrated circuit) is another goal; in this technology clusters would be constructed with electrical properties much like those of transistors and could be packed together to make microcircuits far smaller than any now produced. These applications are still theoretical, however, and have not yet been realized.
Clusters do indeed form a bridge between bulk and molecular matter. Their physical and chemical properties are in many instances unique to their finely divided state. Some examples of clusters, such as the network clusters of carbon, are new forms of matter. Nevertheless, such clusters, particularly the small and middle-size ones, not only exhibit behaviours of their own but also provide new insights into the molecular origins of the properties of bulk matter. They may yield other new materials—e.g., possibly far more disordered, amorphous (amorphous solid) glasslike substances than the glasses now in common use—and at the same time give rise to deeper understanding of why and how glasses form at all.
Additional Reading
Michael A. Duncan and Dennis H. Roubray, “Microclusters,” Scientific American, 261(6):110–115 (December 1989), provides a general introduction and survey for nonscientists. Works presenting the results of recent research include R. Stephen Berry, “When the Melting and Freezing Points Are Not the Same,” Scientific American, 263(2):68–72, 74 (August 1990), written for nonscientists, a description of the melting and freezing of clusters and their relation to bulk melting and freezing; and several collections of conference proceedings: P. Jena, B.K. Rao, and S.N. Khanna (eds.), Physics and Chemistry of Small Clusters (1987), covering a wide variety of topics within cluster science; S. Sugano, Y. Nishina, and S. Ohnishi (eds.), Microclusters (1987); G. Scoles (ed.), The Chemical Physics of Atomic and Molecular Clusters (1990), at the graduate-student level; S. Sugano, Microcluster Physics (1991), accessible to scientifically literate readers; and Zeitschrift für Physik, part D, vol. 19 and 20 (1991) and vol. 26 (1993), the proceedings of the international conferences on small particles and inorganic clusters held in 1990 and 1992, respectively.
- radiation measurement
- radiation pressure
- radiation therapy
- radical
- Radical Civic Union
- Radical Democratic Party
- radical empiricism
- Radical Republican
- Radical-Socialist Party
- Radiguet, Raymond
- Radin, Paul
- radio
- radioactive fallout
- radioactive isotope
- radioactive series
- radioactivity
- radio and radar astronomy
- radio direction finder
- radio-frequency heating
- Radiohead
- radio interferometer
- radiolarian
- radiology
- radiometer
- radio range