steel
metallurgy
Introduction
alloy of iron and carbon in which the carbon content ranges up to 2 percent (with a higher carbon content, the material is defined as cast iron). By far the most widely used material for building the world's infrastructure and industries, it is used to fabricate everything from sewing needles to oil tankers. In addition, the tools required to build and manufacture such articles are also made of steel. As an indication of the relative importance of this material, in 2006 the world's raw steel production was about 1.2 trillion tons, while production of the next most important engineering metal, aluminum, was about 33 million tons. (For a list of steel production by country, see below World steel production (steel).) The main reasons for the popularity of steel are the relatively low cost of making, forming, and processing it, the abundance of its two raw materials (iron ore and scrap), and its unparalleled range of mechanical properties.
Properties of steel
The base metal: iron
The major component of steel is iron, a metal that in its pure state is not much harder than copper. Omitting very extreme cases, iron in its solid state is, like all other metals, polycrystalline—that is, it consists of many crystals that join one another on their boundaries. A crystal is a well-ordered arrangement of atoms that can best be pictured as spheres touching one another. They are ordered in planes, called lattices, which penetrate one another in specific ways. For iron, the lattice arrangement can best be visualized by a unit cube with eight iron atoms at its corners. Important for the uniqueness of steel is the allotropy of iron—that is, its existence in two crystalline forms. In the body-centred cubic (bcc) arrangement, there is an additional iron atom in the centre of each cube. In the face-centred cubic (fcc) arrangement, there is one additional iron atom at the centre of each of the six faces of the unit cube. It is significant that the sides of the face-centred cube, or the distances between neighbouring lattices in the fcc arrangement, are about 25 percent larger than in the bcc arrangement; this means that there is more space in the fcc than in the bcc structure to keep foreign (i.e., alloying) atoms in solid solution.
Iron has its bcc allotropy below 912° C (1,674° F) and from 1,394° C (2,541° F) up to its melting point of 1,538° C (2,800° F). Referred to as ferrite, iron in its bcc formation is also called alpha iron in the lower temperature range and delta iron in the higher temperature zone. Between 912° and 1,394° C iron is in its fcc order, which is called austenite or gamma iron. The allotropic behaviour of iron is retained with few exceptions in steel, even when the alloy contains considerable amounts of other elements.
There is also the term beta iron, which refers not to mechanical properties but rather to the strong magnetic characteristics of iron. Below 770° C (1,420° F), iron is ferromagnetic; the temperature above which it loses this property is often called the Curie point.
Effects of carbon
In its pure form, iron is soft and generally not useful as an engineering material; the principal method of strengthening it and converting it into steel is by adding small amounts of carbon. In solid steel, carbon is generally found in two forms. Either it is in solid solution in austenite and ferrite or it is found as a carbide. The carbide form can be iron carbide (Fe3C, known as cementite), or it can be a carbide of an alloying element such as titanium. (On the other hand, in gray iron, carbon appears as flakes or clusters of graphite, owing to the presence of silicon, which suppresses carbide formation.)

 The effects of carbon are best illustrated by an iron-carbon equilibrium diagram (seefigure-->
The effects of carbon are best illustrated by an iron-carbon equilibrium diagram (seefigure--> ). The A-B-C line represents the liquidus points (i.e., the temperatures at which molten iron begins to solidify), and the H-J-E-C line represents the solidus points (at which solidification is completed). The A-B-C line indicates that solidification temperatures decrease as the carbon content of an iron melt is increased. (This explains why gray iron, which contains more than 2 percent carbon, is processed at much lower temperatures than steel.) Molten steel containing, for example, a carbon content of 0.77 percent (shown by the vertical dashed line in thefigure-->
). The A-B-C line represents the liquidus points (i.e., the temperatures at which molten iron begins to solidify), and the H-J-E-C line represents the solidus points (at which solidification is completed). The A-B-C line indicates that solidification temperatures decrease as the carbon content of an iron melt is increased. (This explains why gray iron, which contains more than 2 percent carbon, is processed at much lower temperatures than steel.) Molten steel containing, for example, a carbon content of 0.77 percent (shown by the vertical dashed line in thefigure--> ) begins to solidify at about 1,475° C (2,660° F) and is completely solid at about 1,400° C (2,550° F). From this point down, the iron crystals are all in an austenitic—i.e., fcc—arrangement and contain all of the carbon in solid solution. Cooling further, a dramatic change takes place at about 727° C (1,341° F) when the austenite crystals transform into a fine lamellar structure consisting of alternating platelets of ferrite and iron carbide. This microstructure is called pearlite, and the change is called the eutectoidic transformation. Pearlite has a diamond pyramid hardness (DPH) of approximately 200 kilograms-force per square millimetre (285,000 pounds per square inch), compared to a DPH of 70 kilograms-force per square millimetre for pure iron. Cooling steel with a lower carbon content (e.g., 0.25 percent) results in a microstructure containing about 50 percent pearlite and 50 percent ferrite; this is softer than pearlite, with a DPH of about 130. Steel with more than 0.77 percent carbon—for instance, 1.05 percent—contains in its microstructure pearlite and cementite; it is harder than pearlite and may have a DPH of 250.
) begins to solidify at about 1,475° C (2,660° F) and is completely solid at about 1,400° C (2,550° F). From this point down, the iron crystals are all in an austenitic—i.e., fcc—arrangement and contain all of the carbon in solid solution. Cooling further, a dramatic change takes place at about 727° C (1,341° F) when the austenite crystals transform into a fine lamellar structure consisting of alternating platelets of ferrite and iron carbide. This microstructure is called pearlite, and the change is called the eutectoidic transformation. Pearlite has a diamond pyramid hardness (DPH) of approximately 200 kilograms-force per square millimetre (285,000 pounds per square inch), compared to a DPH of 70 kilograms-force per square millimetre for pure iron. Cooling steel with a lower carbon content (e.g., 0.25 percent) results in a microstructure containing about 50 percent pearlite and 50 percent ferrite; this is softer than pearlite, with a DPH of about 130. Steel with more than 0.77 percent carbon—for instance, 1.05 percent—contains in its microstructure pearlite and cementite; it is harder than pearlite and may have a DPH of 250.Effects of heat-treating
 Adjusting the carbon content is the simplest way to change the mechanical properties of steel. Additional changes are made possible by heat-treating—for instance, by accelerating the rate of cooling through the austenite-to-ferrite transformation point, shown by the P-S-K line in thefigure-->
Adjusting the carbon content is the simplest way to change the mechanical properties of steel. Additional changes are made possible by heat-treating—for instance, by accelerating the rate of cooling through the austenite-to-ferrite transformation point, shown by the P-S-K line in thefigure--> . (This transformation is also called the Ar1 transformation, r standing for refroidissement, or “cooling.”) Increasing the cooling rate of pearlitic steel (0.77 percent carbon) to about 200° C per minute generates a DPH of about 300, and cooling at 400° C per minute raises the DPH to about 400. The reason for this increasing hardness is the formation of a finer pearlite and ferrite microstructure than can be obtained during slow cooling in ambient air. In principle, when steel cools quickly, there is less time for carbon atoms to move through the lattices and form larger carbides. Cooling even faster—for instance, by quenching the steel at about 1,000° C per minute—results in a complete depression of carbide formation and forces the undercooled ferrite to hold a large amount of carbon atoms in solution for which it actually has no room. This generates a new microstructure, martensite. The DPH of martensite is about 1,000; it is the hardest and most brittle form of steel. tempering martensitic steel—i.e., raising its temperature to a point such as 400° C and holding it for a time—decreases the hardness and brittleness and produces a strong and tough steel. Quench-and-temper heat treatments are applied at many different cooling rates, holding times, and temperatures; they constitute a very important means of controlling steel's properties. (See also below Treating of steel: Heat-treating (steel).)
. (This transformation is also called the Ar1 transformation, r standing for refroidissement, or “cooling.”) Increasing the cooling rate of pearlitic steel (0.77 percent carbon) to about 200° C per minute generates a DPH of about 300, and cooling at 400° C per minute raises the DPH to about 400. The reason for this increasing hardness is the formation of a finer pearlite and ferrite microstructure than can be obtained during slow cooling in ambient air. In principle, when steel cools quickly, there is less time for carbon atoms to move through the lattices and form larger carbides. Cooling even faster—for instance, by quenching the steel at about 1,000° C per minute—results in a complete depression of carbide formation and forces the undercooled ferrite to hold a large amount of carbon atoms in solution for which it actually has no room. This generates a new microstructure, martensite. The DPH of martensite is about 1,000; it is the hardest and most brittle form of steel. tempering martensitic steel—i.e., raising its temperature to a point such as 400° C and holding it for a time—decreases the hardness and brittleness and produces a strong and tough steel. Quench-and-temper heat treatments are applied at many different cooling rates, holding times, and temperatures; they constitute a very important means of controlling steel's properties. (See also below Treating of steel: Heat-treating (steel).)Effects of alloying (alloy)
A third way to change the properties of steel is by adding alloying elements other than carbon that produce characteristics not achievable in plain carbon steel. Each of the approximately 20 elements used for alloying steel has a distinct influence on microstructure and on the temperature, holding time, and cooling rates at which microstructures change. They alter the transformation points between ferrite and austenite, modify solution and diffusion rates, and compete with other elements in forming intermetallic compounds such as carbides and nitrides. There is a huge amount of empirical information on how alloying affects heat-treatment conditions, microstructures, and properties. In addition, there is a good theoretical understanding of principles, which, with the help of computers, enables engineers to predict the microstructures and properties of steel when alloying, hot-rolling, heat-treating, and cold-forming in any way.
A good example of the effects of alloying is the making of a high-strength steel with good weldability. This cannot be done by using only carbon as a strengthener, because carbon creates brittle zones around the weld, but it can be done by keeping carbon low and adding small amounts of other strengthening elements, such as nickel or manganese. In principle, the strengthening of metals is accomplished by increasing the resistance of lattice structures to the motion of dislocations. Dislocations are failures in the lattices of crystals that make it possible for metals to be formed. When elements such as nickel are kept in solid solution in ferrite, their atoms become embedded in the iron lattices and block the movements of dislocations. This phenomenon is called solution hardening. An even greater increase in strength is achieved by precipitation hardening, in which certain elements (e.g., titanium, niobium, and vanadium) do not stay in solid solution in ferrite during the cooling of steel but instead form finely dispersed, extremely small carbide or nitride crystals, which also effectively restrict the flow of dislocations. In addition, most of these strong carbide or nitride formers generate a small grain size, because their precipitates have a nucleation effect and slow down crystal growth during recrystallization of the cooling metal. Producing a small grain size is another method of strengthening steel, since grain boundaries also restrain the flow of dislocations.
Alloying elements have a strong influence on heat-treating, because they tend to slow the diffusion of atoms through the iron lattices and thereby delay the allotropic transformations. This means, for example, that the extremely hard martensite, which is normally produced by fast quenching, can be produced at lower cooling rates. This results in less internal stress and, most important, a deeper hardened zone in the workpiece. Improved hardenability is achieved by adding such elements as manganese, molybdenum, chromium, nickel, and boron. These alloying agents also permit tempering at higher temperatures, which generates better ductility at the same hardness (hardness tester) and strength.
Testing of properties
The testing of steel's properties often begins with checking hardness. This is measured by pressing a diamond pyramid or a hard steel ball into the steel at a specific load. The Vickers Diamond Pyramid Hardness tester, which measures the DPH mentioned above, uses an indenter with an included angle of 136° between opposite faces of a pyramid and usually a load of 10, 30, or 50 kilograms-force. The diagonal of the impression is measured optically, and the hardness expressed as the load in kilograms-force divided by the impressed area of the pyramid in square millimetres. Tensile and yield strength are determined by pulling a standardized machined sample in a special hydraulic press and recording the pulling force at increasing elongations until the sample breaks. The elongation at this point, and the way the fracture looks, are good indications of the steel's ductility. Measuring the pulling force at 0.20 percent elongation and dividing it by the test bar's cross section are a means of calculating the yield strength, a good indicator of cold formability. Impact toughness is determined by hitting a standardized, prismatic, notched sample with a test swing hammer and recording the work required to break it. This is performed at different temperatures, because brittleness increases as temperature falls.
There are many other tests used in the industry to check a steel's mechanical properties, such as wear tests for rails, drawability tests for sheets, and bending tests for wire. Metallographic laboratories examine the microstructure of polished and etched steel samples, often on computerized and very powerful (up to 80,000× magnification) microscopes. Laboratories also check physical data such as thermal elongation and electromagnetic properties. Chemical composition is often checked by completely automated spectrometers. There are also several nondestructive tests as, for example, the ultrasonic test and magnaflux test used to check for internal and external flaws such as laminations or cracks.
Types of steel
There are several thousand steel grades either published, registered, or standardized worldwide, all of which have different chemical compositions, and special numbering systems have been developed in several countries to classify the huge number of alloys. In addition, all the different possible heat treatments, microstructures, cold-forming conditions, shapes, and surface finishes mean that there is an enormous number of options available to the steel user. Fortunately, steels can be classified reasonably well into a few major groups according to their chemical compositions, applications, shapes, and surface conditions.
Chemical composition
On the basis of chemical composition, steels can be grouped into three major classes: carbon steels, low-alloy steels, and high-alloy steels. All steels contain a small amount of incidental elements left over from steelmaking. These include manganese, silicon, or aluminum from the deoxidation process conducted in the ladle, as well as phosphorus and sulfur picked up from ore and fuel in the blast furnace. Copper and other metals, called residuals, are introduced by scrap used in the steelmaking furnace. Because all these elements together normally constitute less than 1 percent of the steel, they are not considered alloys.
Carbon steels are by far the most produced and used, accounting for about 90 percent of the world's steel production. They are usually grouped into high-carbon steels, with carbon above 0.5 percent; medium-carbon steels, with 0.2 to 0.49 percent carbon; low-carbon steels, with 0.05 to 0.19 percent carbon; extra-low-carbon steels, with 0.015 to 0.05 percent carbon; and ultralow-carbon steels, with less than 0.015 percent carbon. Carbon steels are also defined as having less than 1.65 percent manganese, 0.6 percent silicon, and 0.6 percent copper, with the total of these other elements not exceeding 2 percent.
Low-alloy steels have up to 8 percent alloying elements; any higher concentration is considered to constitute a high-alloy steel. There are about 20 alloying elements besides carbon. These are manganese, silicon, aluminum, nickel, chromium, cobalt, molybdenum, vanadium, tungsten, titanium, niobium, zirconium, nitrogen, sulfur, copper, boron, lead, tellurium, and selenium. Several of these are often added simultaneously to achieve specific properties.
Application
The many applications of steel demonstrate best the great versatility of this material. Most often, steel consumers' needs are met by carbon steels. Good examples are sheets for deep-drawn automobile bodies and appliances made of low-carbon steels, medium-carbon structural steels and plates employed in all kinds of construction, high-carbon railroad rails, and wires at all carbon levels used for hundreds of items. The addition of costly alloys begins when combinations of properties are requested that cannot be met by carbon steels.
High-strength low-alloy steels
The demand for high strength, good weldability, and higher resistance to atmospheric corrosion is met by a group called the high-strength low-alloy (HSLA) steels. These grades have low carbon levels (e.g., 0.05 percent) and contain small amounts of one or a combination of elements such as chromium, nickel, molybdenum, vanadium, titanium, and niobium. HSLA steels are used for oil or gas pipelines, ships, offshore structures, and storage tanks.
Free-machining steels
This group, developed for good machinability and fabricated into bolts, screws, and nuts, contains up to 0.35 percent sulfur and 0.35 percent lead; also, it sometimes has small additions of tellurium or selenium. These elements form many inclusions, which are normally avoided but are desired in this application because they break the long, hazardous strings of metal that are usually formed during machining into small chips. This keeps tools and workpieces clean, improves tool life, and permits machining at higher speeds.
Wear-resistant steels
Another group is the wear-resistant steels, made into wear plates for rock-processing machinery, crushers, and power shovels. These are austenitic steels that contain about 1.2 percent carbon and 12 percent manganese. The latter element is a strong austenizer; that is, it keeps steel austenitic at room temperature. Manganese steels are often called Hadfield steels, after their inventor, Robert Hadfield.
Wear resistance is brought about by the high work-hardening capabilities of these steels; this in turn is generated during the pounding (i.e., deforming) of the surface, when a large number of disturbances are created in the lattices of their crystals that effectively block the flow of dislocations. In other words, the more pounding the steel takes, the stronger it becomes. Such significant increases in strength by cold forming are also utilized in the production of high-strength, cold-drawn wire such as those used in prestressed concrete or automobile tires. A special case, piano wire drawn from 0.8-percent-carbon steel, can reach a tensile strength of 275 kilograms-force per square millimetre.
Bearing steels
One important group that well demonstrates the enormous impact of material developments on engineering possibilities is the steels used for roller and ball bearings. These steels often contain 1 percent carbon, 1.2 percent chromium, 0.25 percent nickel, and 0.25 percent molybdenum and are very hard after heat treatment. Most important, however, they are extremely clean, having been purged of practically all inclusions by vacuum treatment of the liquid steel. Inclusions are very harmful in bearings because they create stress concentrations that result in low fatigue strength.
Stainless steels (stainless steel)
This outstanding group receives its stainless characteristics from an invisible, self-healing chromium oxide film that forms when chromium is added at concentrations greater than 10.5 percent. There are three major groups, the austenitic, the ferritic, and the martensitic.
The best corrosion resistance is obtained in austenitic stainless steels. Their microstructures consist of very clean fcc crystals in which all alloying elements are held in solid solution. These steels contain 16 to 26 percent chromium and up to 35 percent nickel, which, like manganese, is a strong austenizer. (Indeed, manganese is sometimes used instead of nickel.) Austenitic steels cannot be hardened by heat treatment; they are also nonmagnetic. The most common type is the 18/8 or 304 grade, which contains 18 percent chromium and 8 percent nickel.
The ferritic and martensitic groups both have a bcc microstructure. The latter has a higher carbon level (up to 1.2 percent); it can be hardened and is used for knives and tools. Ferritic stainless steels contain only up to 0.12 percent carbon. Both types have 11.5 to 29 percent chromium as their main alloy addition and practically no nickel. Their corrosion resistance is modest, and they are ferromagnetic.
A special group of stainless steels is employed at high temperatures—e.g., 800° C (1,450° F). Solution hardening is used in this group to keep the steels strong at such heat. They contain up to 25 percent chromium and 20 percent nickel, in addition to small amounts of strong carbide formers such as niobium or titanium to tie up the carbon and avoid a depletion of chromium at the grain boundaries. For even more severe service, as in aircraft jet engines or gas turbines, superalloys are used. These work on the same principle, but they are based on nickel or cobalt or both and contain either no iron at all or only up to 30 percent iron. Their maximum service temperature can reach 80 percent of their melting point.
Electrical steels
An important group of steels, necessary for the generation and transmission of electrical power, is the high-silicon electrical steels. Electromagnets for alternating current are always made by laminating many thin sheets, which are insulated in order to minimize the flow of eddy currents and thereby reduce current losses and heat generation. A further improvement is achieved by adding up to 4.5 percent silicon, which imparts high electrical resistance. For electric transformers, grain-oriented sheets are often used; these contain about 3.5 percent silicon and are rolled and annealed in such a way that the edges of the unit cubes are oriented parallel to the direction of rolling. This improves the magnetic flux density by about 30 percent.
Tool steels
Tool steels are produced in small quantities, contain expensive alloys, and are often sold only by the kilogram and by their individual trade names. Generally they are very hard, wear-resistant, tough, inert to local overheating, and frequently engineered to particular service requirements. They also have to be dimensionally stable during hardening and tempering. They contain strong carbide formers such as tungsten, molybdenum, vanadium, and chromium in different combinations and often cobalt or nickel to improve high-temperature performance.
Shape and surface
In principle, steel is formed into either flat products or long products, both of which have either a hot-rolled, cold-formed, or coated surface.
Flat products
Flat products include plates, hot-rolled strip and sheets, and cold-rolled strip and sheets; all have a great variety of surface conditions. They are rolled from slabs, which are considered a semifinished product and are normally not sold. Provided by either a continuous caster or rolled from ingots by a slabbing mill, slabs are 50 to 250 millimetres thick, 0.6 to 2.6 metres wide, and up to 12 metres long (that is, 2 to 10 inches thick, 24 to 104 inches wide, and up to 40 feet long).
Plates are hot-rolled either from slabs or directly from ingots. Maximum dimensions vary with available slab sizes or ingot weights and with the sizes of installed rolling mills and auxiliary equipment. Thickness can be as low as 5 millimetres, but it is usually heavier (e.g., 25 millimetres) and can go as high as 200 millimetres. The width of plates is usually between 1.5 to 3.5 metres, but there are plants that can roll plates up to 5.5 metres wide. The maximum plate length that the largest mills can produce is 35 metres. Plates are usually made in small quantities and to a customer's specification, with different dimensions and tolerances for flatness, profile, straightness, and other properties. The edges can be ordered in either as-rolled condition or sheared, machined, or gas-cut. Plates are also sometimes cladded with corrosion-resistant sheets.
Hot-rolled strip is often shipped directly from the hot-strip mill in a large coil weighing 10 to 35 tons. Its thickness is 1.5 to 12 millimetres, and its width, depending on the available mill, is 0.7 to 2 metres. Frequently, the large coils are slit into narrower coils or edge trimmed, or they are cut to length into sheets at the finishing section of a steel plant or at a service centre. Coils and sheets are shipped either with the hot-rolled surface or with the scale removed and the surface oiled.
Cold-rolled strip, produced from hot-rolled strip, is 0.1 to 2 millimetres thick and also up to 2 metres wide, depending on a shop's facilities. Steel plants supply this product in coils or sheets, the latter being cut on special shear lines. Cold-rolled products are available in a great variety of surface conditions, often with a specific roughness, electrolytically cleaned, chemically treated, oiled, or coated with metals such as zinc, tin, chromium, and aluminum or with organic substances. They are usually produced to strict dimensional tolerances in order to assure efficient performance in the highly demanding operations of automated consumer-products industries.
Long products
Long products are made of either blooms or billets, which are, like slabs, considered a semifinished product and are cast by a continuous caster or rolled at a blooming mill. Billets have a cross section 50 to 125 millimetres square, and blooms are 125 to 400 millimetres square. In practice, they are not precisely distinguished by these dimensions, and there is considerable overlap in the use of the two terms.
Long products include bars, rods and wires, structural shapes and rails, and tubes. Bars are long products with square, rectangular, flat, round, hexagonal, or octagonal cross sections. The most important bar products are the rounds, which can reach a diameter of 250 millimetres. They are sometimes cold-drawn or even ground to very precise dimensions for use in machine parts. A special group of rounds are the reinforcing bars. Produced in diameters of 10 to 50 millimetres, they provide tensile strength to concrete sections subjected to a bending load. They normally have hot-rolled protrusions on their surface to improve bonding with concrete. Some bar mills also produce small channels, angles, tees, zees, and fence-post sections, with a maximum flange length of 75 millimetres, and call these products merchant bars.
Hot-rolled wire rods are produced in diameters between 5.5 and 12.5 millimetres and are shipped in coils weighing up to two tons. A great portion of these rods are cold-drawn into wire, which is often covered afterward by a metallic coating for corrosion protection. The use of wire is extremely wide, ranging from cords for belted tires to cables for suspension bridges.
The common structural shapes are wide flange I-beams, standard I-beams, channels, angles, tees, zees, H-pilings, and sheet pilings. All these shapes are standardized, and each company has price lists showing which sections are produced and in which quality and length they can be supplied. Railroad rails are always produced to national standards. In the United States, for example, there are rails weighing 115, 132, and 140 pounds per yard and cut to lengths of 39 or 78 feet. There are also a great number of special rails—e.g., for cranes and heavy transfer cars or for use in mines and construction.
Tubular steels are broadly grouped into welded and seamless products. Longitudinally welded tubes are normally produced up to 500 millimetres in diameter and 10 millimetres in wall thickness. Pipes produced from heavy plates are also longitudinally welded after being formed in a U-ing and O-ing operation; they can be 0.8 to 2 metres in diameter, with wall thicknesses up to 180 millimetres. Spiral-welded pipes are sometimes produced in diameters up to 1.5 metres. Seamless tubes are subjected to more demanding service; they are often rolled in diameters ranging from 120 to 400 millimetres and in wall thicknesses up to 15 millimetres, although special rolling mills can often increase the diameter to 650 millimetres. Smaller diameter tubes, both welded and seamless, can be produced by reduction mills or cold-drawing benches. Tubes are frequently machined on both ends for various coupling systems and coated with organic material.
Standards
Specifications for steel products as well as testing procedures are normally included in the general standard systems of most industrial countries. Institutions providing these standards are the American Society for Testing and Materials, Philadelphia; British Standards Institute, London; Deutsches Institut für Normung, Berlin; Japanese Industrial Standards Committee, Tokyo; Comité Européen de Normalisation, Brussels; and International Organization for Standardization, Geneva.
There are also product manuals published by a number of associations and societies, sometimes for special products only, that are often used as standards in technical specifications and commercial agreements. Organizations that issue these include the American Iron and Steel Institute, Washington, D.C.; Society of Automotive Engineers, Warrendale, Pa.; American Petroleum Institute, Washington, D.C.; and American Society of Mechanical Engineers, New York City.
Each steel producer publishes lists showing the steel grades and dimensions that it can deliver. Special alloys and coatings are often supplied under a company-owned trademark. There are also publications that provide cross-references for similar steel grades among the various standards and trademarks issued in different countries.
Primary steelmaking
Principles
In principle, steelmaking is a melting, purifying, and alloying process carried out at approximately 1,600° C (2,900° F) in molten conditions. Various chemical reactions are initiated, either in sequence or simultaneously, in order to arrive at specified chemical compositions and temperatures. Indeed, many of the reactions interfere with one another, requiring the use of process models to help in analyzing options, optimizing competing reactions, and designing efficient commercial practices.
Raw materials
The major iron-bearing raw materials for steelmaking are blast-furnace iron, steel scrap, and direct-reduced iron (DRI). Liquid blast-furnace iron typically contains 3.8 to 4.5 percent carbon (C), 0.4 to 1.2 percent silicon (Si), 0.6 to 1.2 percent manganese (Mn), up to 0.2 percent phosphorus (P), and 0.04 percent sulfur (S). Its temperature is usually 1,400° to 1,500° C (2,550° to 2,700° F). The phosphorus content depends on the ore used, since phosphorus is not removed in the blast-furnace process, whereas sulfur is usually picked up during iron making from coke and other fuels. DRI is reduced from iron ore in the solid state by carbon monoxide (CO) and hydrogen (H2). It frequently contains about 3 percent unreduced iron ore and 4 percent gangue, depending on the ore used. It is normally shipped in briquettes and charged into the steelmaking furnace like scrap. Steel scrap is metallic iron containing residuals, such as copper, tin, and chromium, that vary with its origin. Of the three major steelmaking processes—basic oxygen, open hearth, and electric arc—the first two, with few exceptions, use liquid blast-furnace iron and scrap as raw material and the latter uses a solid charge of scrap and DRI.
Oxidation reactions
The most important chemical reactions carried out on these materials (especially on blast-furnace iron) are the oxidation of carbon to carbon monoxide, silicon to silica, manganese to manganous oxide, and phosphorus to phosphate, as follows:

Unfortunately, iron is also lost in this series of reactions, as it is oxidized to ferrous oxide:

The FeO, absorbed into the liquid slag, then acts as an oxidizer itself, as in the following reactions:

In the open-hearth furnace, oxidation also takes place when gases containing carbon dioxide (CO2) contact the melt and react as follows:

The slag
The products of the above reactions, the oxides silica, manganese oxide, phosphate, and ferrous oxide, together with burnt lime (calcium oxide; CaO) added as flux, form the slag. Burnt lime has by itself a high melting point of 2,570° C (4,660° F) and is therefore solid at steelmaking temperatures, but when it is mixed with the other oxides, they all melt together at lower temperatures and thus form the slag. A basic slag contains approximately 55 percent CaO, 15 percent SiO2, 5 percent MnO, 18 percent FeO, and other oxides plus sulfides and phosphates. The basicity of a slag is often simply expressed by the ratio of CaO to SiO2, with CaO being the basic and SiO2 the acidic component. Usually, a basicity above 3.5 provides good absorption and holding capacity for calcium phosphates and calcium sulfides.
Removing sulfur
The majority of sulfur, present as ferrous sulfide (FeS), is removed from the melt not by oxidation but by the conversion of calcium oxide to calcium sulfide:
FeS + CaO → CaS + FeO.
According to this equation, desulfurization is successful only when using a slag with plenty of calcium oxide—in other words, with a high basicity. A low iron oxide content is also essential, since oxygen and sulfur compete to combine with the calcium. For this reason, many steel plants desulfurize blast-furnace iron before it is refined into steel, since at that stage it contains practically no dissolved oxygen, owing to its high silicon and carbon content. Nevertheless, sulfur is often introduced by scrap and flux during steelmaking, so that, in order to meet low sulfur specifications (for example, less than 0.008 percent), it is necessary to desulfurize the steel as well.
Removing carbon
A very important chemical reaction during steelmaking is the oxidation of carbon. Its gaseous product, carbon monoxide, goes into the off-gas, but, before it does that, it generates the carbon monoxide boil, a phenomenon common to all steelmaking processes and very important for mixing. Mixing enhances chemical reactions, purges hydrogen and nitrogen, and improves heat transfer. Adjusting the carbon content is important, but it is often oxidized below specified levels, so that carbon powder must be injected to raise the carbon again.
Removing oxygen
As the carbon level is lowered in liquid steel, the level of dissolved oxygen theoretically increases according to the relationship %C × %O = 0.0025. This means that, for instance, a steel with 0.1 percent carbon, at equilibrium, contains about 0.025 percent, or 250 parts per million, dissolved oxygen. The level of dissolved oxygen in liquid steel must be lowered because oxygen reacts with carbon during solidification and forms carbon monoxide and blowholes in the cast. This reaction can start earlier, too, resulting in a dangerous carbon monoxide boil in the ladle. In addition, a high oxygen level creates many oxide inclusions that are harmful for most steel products. Therefore, usually at the end of steelmaking during the tapping stage, liquid steel is deoxidized by adding aluminum or silicon. Both elements are strong oxide formers and react with dissolved oxygen to form alumina (Al2O3) or silica. These float to the surface of the steel, where they are absorbed by the slag. The upward movement of these inclusions is often slow because they are small (e.g., 0.05 millimetre), and combinations of various deoxidizers are sometimes used to form larger inclusions that float more readily. In addition, stirring the melt with argon or an electromagnetic field often serves to give them a lift.
Alloying (alloy)
Deoxidation is also important before alloying steel with easy oxidizable metals such as chromium, titanium, and vanadium, in order to minimize losses and improve process control. Metals that do not oxidize readily, such as nickel, cobalt, molybdenum, and copper, can be added in the furnace to take advantage of high heating rates. In fact, alloying always has thermal effects on steelmaking—for example, the use of energy to heat and melt the alloying agents, or the heat of reaction or solution when they combine with other elements. Fortunately, there exists a large amount of empirical data, obtained from thousands of thermodynamic experiments, that, when supported by theoretical principles, allows steelmakers to predict such temperature changes.
Most alloys are added in the form of ferroalloys (ferroalloy), which are iron-based alloys that are cheaper to produce than the pure metals. Many different grades are available. For example, ferrosilicon is supplied with levels of 50, 75, and 90 percent silicon and with varying levels of carbon and other additions.
Removing hydrogen and nitrogen
Also important for steelmaking is the absorption and removal of the two gases hydrogen and nitrogen. Hydrogen can enter liquid steel from moist air, damp refractories, and wet flux and alloy additions. It causes brittleness of solidified steel—especially in large pieces, such as heavy forgings, that do not permit the gas to diffuse to the surface. Hydrogen can also form blowholes in castings. Nitrogen does not move into and out of liquid steel as easily as hydrogen, but it is well absorbed by liquid steel in the high-temperature zones of an electric arc or oxygen jet, where nitrogen molecules (N2) are broken up into atoms (N). Like hydrogen, nitrogen substantially decreases the ductility of steel.
refractory liner
Basic steelmaking takes place in containers lined with basic refractories. These may be bricks or ram material made of highly stable oxides, such as magnesite, alumina, or the double oxides chrome-magnesite and dolomite. It is desirable that the refractories not participate in the steelmaking reactions, but unfortunately they do erode and corrode. Refractory bricks are produced in all shapes and grades by a highly specialized industry.
Testing
Testing and sampling are an important part of liquid steelmaking. They are carried out by mechanized and often automated facilities, which immerse lances that are equipped with sensors for rapid computation of temperature and dissolved carbon, oxygen, and hydrogen. Test lances also take samples for analysis in laboratories. All results are usually fed automatically into a process-control computer.
Basic oxygen steelmaking
 More than half the world's steel is produced in the basic oxygen process (BOP), which uses pure oxygen to convert a charge of liquid blast-furnace iron and scrap into steel. The basic oxygen furnace (BOF) is a refractory-lined, tiltable converter into which a vertically movable, water-cooled lance is inserted to blow oxygen through nozzles at supersonic velocity onto the charge (see figure-->
More than half the world's steel is produced in the basic oxygen process (BOP), which uses pure oxygen to convert a charge of liquid blast-furnace iron and scrap into steel. The basic oxygen furnace (BOF) is a refractory-lined, tiltable converter into which a vertically movable, water-cooled lance is inserted to blow oxygen through nozzles at supersonic velocity onto the charge (see figure--> ). The use of pure oxygen at high flow rates results in such fast oxidation of the elements contained in blast-furnace iron that only about 20 minutes are required per heat—i.e., to refine one charge. Converters vary in size and are operated for heats ranging from 30 to 360 tons.
). The use of pure oxygen at high flow rates results in such fast oxidation of the elements contained in blast-furnace iron that only about 20 minutes are required per heat—i.e., to refine one charge. Converters vary in size and are operated for heats ranging from 30 to 360 tons.The charge
When oxygen contacts blast-furnace iron, a great amount of heat is released by the ensuing exothermic reactions, especially the oxidation of silicon to silica, so that using only blast-furnace iron would result in a liquid steel temperature too high for casting. Therefore, before the hot metal is added, a specific amount of scrap is charged into the furnace. Melting this scrap consumes about 340 kilocalories per kilogram, effectively cooling the process. A typical BOP charge, therefore, consists of about 75 percent liquid iron and 25 percent scrap. This requires a reliable supply of low-cost iron with a uniform chemical composition, which is attainable only by keeping the operating condition of a blast furnace as constant as possible; this in turn requires a consistent iron consumer. There are also certain iron properties—for example, the silicon and sulfur content—that are selected to optimize the blast furnace and BOF operations and to produce steel at minimal cost. Such interdependence requires that blast furnaces and BOFs work within a well-integrated operating system.
The furnace
The basic oxygen converter is a cylindrical vessel with an open cone on top. For the largest converters, those that make 360-ton heats, the shell is about 8 metres in diameter and 11 metres high. The shells are built of heavy steel plates and sit in a trunnion ring so that the converter may be rotated for charging, testing, tapping, and slag-off. The lining, normally made of magnesite bricks, has different thickness and brick quality in certain zones, depending on the wear at each location. Total lining thickness of large converters exceeds one metre. The taphole is in the upper zone of the converter, right under the cone.
 Oxygen lances are large, multiwall tubes that, on large converters, are about 300 millimetres in diameter and 21 metres long. Their tips have three to five nozzles, directed slightly outward, which produce the supersonic jets of oxygen. Proper water cooling of these lances is crucial. Special lance cranes (seefigure-->
Oxygen lances are large, multiwall tubes that, on large converters, are about 300 millimetres in diameter and 21 metres long. Their tips have three to five nozzles, directed slightly outward, which produce the supersonic jets of oxygen. Proper water cooling of these lances is crucial. Special lance cranes (seefigure--> ) move the lance up and down and adjust its distance from the steel bath. The lances last for about 150 heats before their tips have to be replaced.
) move the lance up and down and adjust its distance from the steel bath. The lances last for about 150 heats before their tips have to be replaced.BOFs are equipped with huge off-gas systems in order to avoid gas leakage into the shop and to ensure proper cleaning of the gases before they are discharged into the atmosphere. Off-gas emerges from the converter mouth at about 1,650° C (3,000° F). It consists of about 90 percent carbon monoxide and 10 percent carbon dioxide, and it also contains ferrous oxide dust, which forms in the high-temperature zone of the oxygen jet. Two off-gas systems are in use: the full combustion and the suppressed combustion.
In the full-combustion system, off-gas is burned above the mouth of the converter with excess air, and both physical and chemical heat are utilized in a boiler or hot-water system incorporated in the hood and vertical offtakes. A large venturi scrubber or electrostatic precipitator then cleans the cooled off-gas. During the blow of a large converter, about 10,000 cubic metres (350,000 cubic feet) of off-gas is moved per minute through full-combustion apparatus by exhaust fans, and about 0.7 kilogram of iron oxide dust is collected per ton of steel.
In the other system, the suppressed-combustion system, a ring-shaped hood is lowered onto the converter mouth before the blow, keeping air away from the hot off-gases. This means that they are not burned and that their chemical heating value of about 3,000 kilocalories per cubic metre is preserved. The gas is cleaned, collected in gas holders, and used at other locations. Though this system is more complicated, it is much smaller, because off-gases are cooler and there is less to be handled and processed.
BOFs are housed in huge buildings sometimes 80 metres high to accommodate the long lance, the off-gas system, and gravity-type feeding equipment. Heavy cranes, long conveyor belts, and railroad tracks assure prompt supply of raw material to the converters and fast removal of liquid steel and slag from the BOF.
The process
Making a heat begins with an inspection of the refractory lining, with the converter in a turned-down position. Sometimes a laser contour instrument is used to determine the remaining lining thickness. With the converter tilted at about 45°, scrap is then charged into the furnace by heavy cranes or special charging machines that drop one or two large boxes full of scrap through the converter mouth. Hot metal is poured into the converter by a special iron-charging ladle; this ladle receives the iron at a transfer station from transport ladles, which bring the iron from the blast furnace. Many plants lower the sulfur content of the iron just before it is charged into the converter by injecting a lime-magnesium mixture or calcium carbide or both into the charging ladle. Any blast-furnace slag and slag formed during desulfurization is skimmed off before the iron is charged.
Owing to predictable losses during the oxygen blow, there is always more iron and scrap charged than steel produced; for example, 1,080 kilograms of raw material may yield 1,000 kilograms of liquid steel, for a metallic yield of 92.6 percent. Chemical compositions, temperatures, and charging weights of the iron are often fed automatically into a control computer. For blowing, the converter is placed in an upright position, oxygen is turned on, and the lance is lowered. Oxygen flow rates, lance height, and lime additions are often controlled automatically. The flow rates of oxygen at large converters exceed 800 cubic metres per minute, and oxygen consumption is about 110 cubic metres per ton of steel. Usually, about 70 kilograms of pebble-sized burnt lime is added per ton of steel early in the blow; this combines with silica and other oxides to form about 150 kilograms of slag per ton of steel. Adding burnt dolomite (CaO·MgO) results in a magnesia (MgO) content in the slag of about 6 percent, thereby decreasing slag corrosion of the magnesite lining. Lime quality is of great importance in BOF operations, and special lime kilns are used to burn a high grade of limestone.
The oxidation reactions in the converter become violent at the highest rate of carbon removal—that is, when all the silicon is gone—about eight minutes into the blow. At this point oxygen reacts mainly with carbon to generate large amounts of carbon monoxide gas, which mixes with the slag. Keeping the foamy slag from overflowing the converter at the high blowing rates is an important control task. Often a small, water-cooled sensor lance, called the sublance, is immersed into the liquid steel during the end phase of the blow to check and sample the steel. Test results are automatically fed into a control computer, which predicts the end point and shuts off the oxygen when temperature and chemical composition have reached the specified level.
 Well-controlled charging conditions make it possible to tap the heat based only on the sublance test. In other cases, the converter must be turned down and the temperature and chemical composition checked manually. Sometimes burnt lime is added and a short reblow is applied in order to increase the temperature or correct the chemical composition. For tapping, the converter is rotated, and steel is poured through the taphole into a ladle sitting on a transfer car beneath the converter. The temperature of the steel at tapping is specifically selected to fit within a temperature “window” for ingot pouring or continuous casting and after all temperature losses expected during treating and holding of the steel in the ladle have been predicted. For example, a 0.1-percent-carbon steel may tap at 1,596° C, 80° C above its theoretical solidification point. Higher carbon steels would be tapped at lower temperatures, following the A-B-C liquidus line of the equilibrium diagram in thefigure-->
Well-controlled charging conditions make it possible to tap the heat based only on the sublance test. In other cases, the converter must be turned down and the temperature and chemical composition checked manually. Sometimes burnt lime is added and a short reblow is applied in order to increase the temperature or correct the chemical composition. For tapping, the converter is rotated, and steel is poured through the taphole into a ladle sitting on a transfer car beneath the converter. The temperature of the steel at tapping is specifically selected to fit within a temperature “window” for ingot pouring or continuous casting and after all temperature losses expected during treating and holding of the steel in the ladle have been predicted. For example, a 0.1-percent-carbon steel may tap at 1,596° C, 80° C above its theoretical solidification point. Higher carbon steels would be tapped at lower temperatures, following the A-B-C liquidus line of the equilibrium diagram in thefigure--> .
.Aluminum or ferrosilicon are added to the ladle before or during the tap in order to lower the level of dissolved oxygen in steel. Ferromanganese is also added, since most of the manganese content of the blast-furnace iron is oxidized during the blow, leaving only about 0.1 percent in the steel—usually not enough to meet specifications.
When slag appears, the converter is rotated all the way back, and the slag is poured over the converter mouth into a slag pot. For better separation of slag from liquid steel, special taphole-closing devices such as refractory balls or nitrogen jets, as well as slag-detection devices, are often used.
BOFs have a tap-to-tap time of 30 to 45 minutes and can blow more than 30 heats per day. Large BOF shops with three converters can produce up to five million tons of liquid steel per year. Repair and maintenance are extremely important, because steel is made around the clock and there is normally only one maintenance shift per week. A converter lining lasts 1,500 to 3,000 heats, after which it is broken out and a new one installed in a mechanized bricklaying operation. Converter relining takes less than one week.
Variations
There are a number of significant improvements, modifications, and process changes of the BOF steelmaking system. For example, when high-phosphorus ore is smelted in the blast furnace, and the BOF is consequently charged with a liquid iron containing more than 0.15 percent of that element, the LD-AC process can be followed, in which lime powder is injected through the lance along with oxygen for quick slag formation. A two-slag practice is then followed for sufficient phosphorus removal, with the first slag runoff being sold for fertilizer. Another variation that finds wide application is the injecting of argon (or sometimes nitrogen) into the molten charge through permeable refractory blocks in the bottom of the converter. Bottom stirring enhances chemical reactions and lowers the steel temperature at the oxygen impact area, resulting in less oxidation of iron and better yield. Another system, called the Q-BOP, uses no top lance at all, blowing oxygen, burnt-lime powder, and, when needed, argon upward through the liquid melt from several gas-cooled or oil-cooled bottom tuyeres. These tuyeres are two concentric steel tubes, with oxygen flowing from the inside annulus and gas or oil flowing through the outer annulus. Cooling of the tubes is accomplished by the endothermic heat required to break down the natural gas or oil into carbon monoxide and hydrogen.
The service life of the bottom of the Q-BOP converter is lower than that of the side wall, thus demanding additional maintenance time for bottom changing. On the other hand, bottom blowing has the advantage of generating a large contact surface among all reactants, thus improving metallurgical reactions and process control. Yield is also higher, since there is less local iron oxidation. However, less oxidation also means the release of less exothermic heat; this decreases the quantity of scrap that can be charged, which can be a cost disadvantage when the price of scrap is low. For this reason, some steel plants enhance bottom blowing with a postcombustion top lance. This is an oxygen lance with additional ports at the tip for burning carbon monoxide into carbon dioxide inside the converter. The additional heat generated by this combined blowing practice increases the potential scrap-charging rate.
Another technology for increasing scrap rates uses an oxy-fuel lance, which preheats the scrap in the converter for about 20 minutes before the liquid blast-furnace iron is added. Another scrap-increasing practice adds aluminum to the charge or melt; this releases heat as it is burned during the oxygen blow. Still another process injects coal powder through a modified oxygen lance or through special bottom tuyeres, simultaneously applying additional oxygen and using a postcombustion lance. In trial operations, this combination has resulted in scrap-charging capabilities all the way up to 100 percent; in other words, no hot metal has been charged, and the converter has become a scrap melter. Increasing scrap-charging rates helps to keep the plant operating when the supply of blast-furnace iron is limited, as, for example, during a blast-furnace reline.
Electric-arc steelmaking
About one-quarter of the world's steel is produced by the electric-arc method, which uses high-current electric arcs to melt steel scrap and convert it into liquid steel of a specified chemical composition and temperature. External arc heating permits better thermal control than does the basic oxygen process, in which heating is accomplished by the exothermic oxidation of elements contained in the charge. This allows larger alloy additions to be made than are possible in basic oxygen steelmaking. However, electric-arc steelmaking is not as oxidizing, and slag-metal mixing is not as intense; therefore, electric-arc steels normally have carbon contents higher than 0.05 percent. In addition, they usually have a higher nitrogen content of 40 to 120 parts per million, compared with 30 to 50 parts per million in basic-oxygen steels. Nitrogen, which renders steel brittle, is absorbed by liquid steel from air in the high-temperature zone of the arc. The nitrogen content can be lowered by blowing other gases into the furnace, by heating with a short arc, and by applying a vigorous carbon monoxide boil or argon stir to the melt.
The charge
The major charge material of electric-arc steelmaking is scrap steel, and its availability at low cost and proper quality is essential. The importance of scrap quality becomes apparent when making steels of high ductility, which must have a total maximum content of residuals (i.e., copper, chromium, nickel, molybdenum, and tin) of 0.2 percent. Most of these residuals are present in scrap and, instead of oxidizing during steelmaking, they accumulate and increase in recycled scrap. In such cases some shops augment their scrap charges with direct-reduced iron or cold blast-furnace iron, which do not contain residuals. Generally, the higher contents of carbon, nitrogen, and residuals make the electric-arc process less attractive for producing low-carbon, ductile steels.
Most scrap yards keep various grades of scrap separated. High-alloy shops, such as stainless-steel producers, accumulate, purchase, and charge scrap of similar composition to the steel they make in order to minimize expensive alloying additions.
The furnace

 The electric- arc furnace (EAF) is a squat, cylindrical vessel made of heavy steel plates. It has a dish-shaped refractory hearth and three vertical electrodes that reach down through a dome-shaped, removable roof (see figure-->
The electric- arc furnace (EAF) is a squat, cylindrical vessel made of heavy steel plates. It has a dish-shaped refractory hearth and three vertical electrodes that reach down through a dome-shaped, removable roof (see figure--> ). The shell diameter of a 10-, 100-, and 300-ton EAF is approximately 2.5, 6, and 9 metres. The shell sits on a hydraulically operated rocker that tilts the furnace forward for tapping and backward for slag removal. The bottom—i.e., the hearth—is lined with tar-bonded magnesite bricks and has on one side a slightly inclined taphole and a spout or, as shown in thefigure-->
). The shell diameter of a 10-, 100-, and 300-ton EAF is approximately 2.5, 6, and 9 metres. The shell sits on a hydraulically operated rocker that tilts the furnace forward for tapping and backward for slag removal. The bottom—i.e., the hearth—is lined with tar-bonded magnesite bricks and has on one side a slightly inclined taphole and a spout or, as shown in thefigure--> , an oval hearth and a vertical taphole. With this latter arrangement, a furnace needs be tilted only 10° for tapping, producing a tight and short tap stream that decreases heat loss and reoxidation of the liquid steel. Before charging, the vertical taphole is closed from the outside by a movable bottom plate and is filled with refractory sand.
, an oval hearth and a vertical taphole. With this latter arrangement, a furnace needs be tilted only 10° for tapping, producing a tight and short tap stream that decreases heat loss and reoxidation of the liquid steel. Before charging, the vertical taphole is closed from the outside by a movable bottom plate and is filled with refractory sand.Most furnace walls are made of replaceable, water-cooled panels; these are covered inside by sprayed-on refractories and slag for protection and to keep heat loss down. The roof is also made of water-cooled panels and has three circular openings, equally spaced, for insertion of the cylindrical electrodes. Another large roof opening, the so-called fourth hole, is used for off-gas removal. Additional openings in the furnace wall, with water-cooled doors, are used for lance injection, sampling, testing, inspection, and repair. The roof and electrodes can be lifted and moved away for charging scrap and for hearth maintenance.
The graphite electrodes, produced to high standards by a specialized industry, are actually strings of individual electrodes bolted end to end by short graphite nipples. This is done because shorter electrodes are easier to manufacture, transport, and handle. Electrode diameters depend on furnace size; a 100-ton EAF typically uses 600-millimetre electrodes. Three electrode strings are each clamped to arms that extend over the furnace roof and that are bolted to a vertically movable mast located beside the furnace. The mast controls the distance between each electrode tip and the scrap or melt, thereby regulating the arc length and current flow. Power-supply equipment—normally a step-down transformer, vacuum circuit breakers, a tap changer for electrode voltage control, and a furnace transformer—is installed in a concrete vault a short distance from the furnace. Heavy water-cooled cables and the power-carrying arms connect the furnace transformer with the electrodes.
EAF plants are smaller and less expensive to build than integrated steelmaking plants, which, in addition to basic oxygen furnaces, contain blast furnaces, sinter plants, and coke batteries for the making of iron. EAFs are also cost-efficient at low production rates—e.g., 150,000 tons per year—while basic oxygen furnaces and their associated blast furnaces can pay for themselves only if they produce more than 2,000,000 tons of liquid steel per year. Moreover, EAFs can be operated intermittently, while a blast furnace is best operated at very constant rates. The electric power used in EAF operation, however, is high, at 360 to 600 kilowatt-hours per ton of steel, and the installed power system is substantial. A 100-ton EAF often has a 70-megavolt-ampere transformer.
The process
After tapping a heat, the roof is moved away, and the hearth is inspected and, when necessary, repaired. An overhead crane then charges the furnace with scrap from a cylindrical bucket that is open on the top for loading and fitted with a drop bottom for quick charging. Scrap buckets are loaded in such a manner as to assure a cushioning of heavy scrap when the load drops onto the hearth in order to obtain good electrical conductivity in the charge, low risk of electrode breakage, and good furnace wall protection during meltdown. Carbon and slag formers are sometimes added to the charge to prevent overoxidation of the steel and to quicken slag formation. After charging one bucket, the roof is moved back to the furnace, and the electrodes are lowered. Meltdown begins with a low power setting until the electrodes have burned themselves into the light scrap on top of the charge, protecting the sidewalls from overheating during higher-power meltdown. Leaving some scrap unmelted at the furnace wall for its protection, a second bucket is charged and the same meltdown procedure is followed. Melting very light scrap sometimes requires the charging of a third or even fourth bucket.
After meltdown, the carbon level in the steel is about 0.25 percent above the final tap level, which prevents overoxidation of the melt. By this time a basic slag has formed, typically consisting of 55 percent lime, 15 percent silica, and 15 to 20 percent iron oxide. Slag foaming is often generated by injecting carbon or a lime-carbon mixture, which reacts with the iron oxide in the slag to produce carbon monoxide gas. This foam shields the sidewall and permits a higher power setting. As required, the carbon content of the steel is either decreased by oxygen blowing or increased by carbon injection. Samples are taken, the temperature is checked, additions are made, and, when all conditions are right, the furnace is tapped by rotating it forward so that the steel flows over the spout or through the vertical taphole into a ladle. When slag appears, a quick back tilt is applied and the slag is poured through the rear door of the furnace into a slag pot. Some shops leave 15 percent of the liquid steel in the furnace. This “hot heel” practice permits complete slag separation.
Very clean steel—i.e., with low oxygen and sulfur content—can be produced in the EAF by a two-slag practice. After removal of slag from the first oxidizing meltdown, new slag formers are added that contain carbon or aluminum or both as reducing agents. The new reducing slag may consist of 65 percent lime, 20 percent silica, calcium carbide or alumina (or all three), and practically no iron oxide. Alloys, which oxidize easily, are added at this time to minimize losses and to improve metallurgical control. Refining continues under the reducing slag until the heat is ready for tapping. Total heat time is one to four hours, depending on the type of steel made—that is, on the amount of refining applied and auxiliary heating used. Many shops do not apply a two-slag practice but treat the steel, after scrap meltdown and tapping, in ladle treatment stations. These secondary metallurgical plants, discussed below, allow the EAF to run only as a highly efficient scrap melter.
From time to time, as the arc erodes their tips and the high-temperature furnace atmosphere oxidizes their bodies, new electrodes are added to the top of the electrode strings at the furnace. Electrodes are consumed at the rate of three to six kilograms per ton of steel, depending on the type of operation.
Variations
In order to lower power consumption, scrap can be preheated in both batch and continuous processes, often utilizing the heat of furnace off-gases. Scrap preheating to 500° C (930° F) cuts power consumption by 40 to 50 kilowatt-hours per ton, and decreases tap-to-tap time and electrode consumption. Sometimes scrap is preheated inside the EAF by oxyfuel burners, but this requires a large off-gas system for handling combustion gases. In addition, for better mixing and heat transfer, electromagnetic coils or permeable refractory blocks for gas stirring are often installed in furnace bottoms. Applying these methods and using the EAF as a scrap melter can reduce power and electrode consumption to a mere 360 kilowatt-hours per ton and three kilograms per ton. Heat times are reduced to about one hour. This means, by applying methods originally developed for the basic oxygen process, the EAF can approach the steelmaking rates of the BOF.
Several EAFs are operated by direct current (DC) instead of alternating current (AC). DC furnaces normally have only one very large electrode extending through the centre of the roof, with the counter electrode embedded in the furnace bottom and contacting the melt. A hot heel is kept in the furnace to ensure a good current flow through the charge. Power and electrode consumption is lower than in regular AC furnaces. The DC arc has a steadier and quieter burn, which results in less disturbance of the surrounding power system and less noise around the furnace. The electrical equipment is smaller but still expensive because of the required rectifiers. Critical in DC furnace operation are the short life of the bottom electrode, integrity of the hearth, and current limitations with a one-electrode system. Furnaces with capacities up to 130 tons are in operation.
Open-hearth steelmaking
Though it has been almost completely replaced by BOF and EAF steelmaking in many highly industrialized countries, the open hearth nevertheless accounts for about one-sixth of all steel produced worldwide.
The furnace
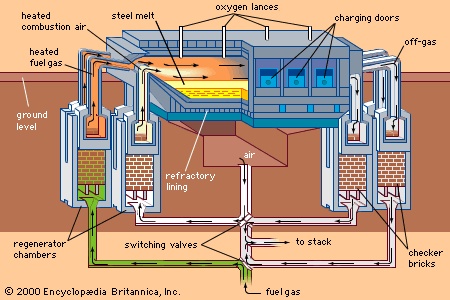 The open-hearth furnace (OHF) uses the heat of combustion of gaseous or liquid fuels to convert a charge of scrap and liquid blast-furnace iron to liquid steel. The high flame temperature required for melting is obtained by preheating the combustion air and, sometimes, the fuel gas. Preheating is done in large, stovelike regenerators or checker chambers, located beneath the furnace (see figure-->
The open-hearth furnace (OHF) uses the heat of combustion of gaseous or liquid fuels to convert a charge of scrap and liquid blast-furnace iron to liquid steel. The high flame temperature required for melting is obtained by preheating the combustion air and, sometimes, the fuel gas. Preheating is done in large, stovelike regenerators or checker chambers, located beneath the furnace (see figure--> ). These contain checker bricks stacked in such a way that they absorb heat from furnace off-gases as they are directed through the chamber. After one chamber has been heated for about 20 minutes, a sliding valve is activated, directing the off-gases to the other chamber and simultaneously bringing air into the heated chamber. This combustion air, after picking up the heat from the checker brick, then enters the furnace through an end-wall above the checker chamber and burns the fuel, which also enters the furnace at the same wall. The combustion flames heat the charge, and the off-gases, after moving across the hearth to the other end wall, are directed downward to heat the other chamber. This cycle, with entry ports becoming exit ports, is reversed every 15 to 20 minutes. After heating the regenerator, the off-gases flow through a heat-recovery boiler and a gas-cleaning system before they are discharged into the atmosphere through a stack.
). These contain checker bricks stacked in such a way that they absorb heat from furnace off-gases as they are directed through the chamber. After one chamber has been heated for about 20 minutes, a sliding valve is activated, directing the off-gases to the other chamber and simultaneously bringing air into the heated chamber. This combustion air, after picking up the heat from the checker brick, then enters the furnace through an end-wall above the checker chamber and burns the fuel, which also enters the furnace at the same wall. The combustion flames heat the charge, and the off-gases, after moving across the hearth to the other end wall, are directed downward to heat the other chamber. This cycle, with entry ports becoming exit ports, is reversed every 15 to 20 minutes. After heating the regenerator, the off-gases flow through a heat-recovery boiler and a gas-cleaning system before they are discharged into the atmosphere through a stack. The OHF itself consists of a shallow, rectangular hearth that holds the charge, liquid steel, and slag (seefigure-->
The OHF itself consists of a shallow, rectangular hearth that holds the charge, liquid steel, and slag (seefigure--> ). Depending on the furnace size, the long front wall on the charging side usually has three to seven rectangular openings fitted with water-cooled doors. These are used for charging scrap and iron, adding flux and alloying agents, running off slag, conducting tests, and repairing the hearth refractory. On the opposite side of the furnace, at the back wall, is the taphole and a spout for tapping into one or two ladles. The two end walls are used as inlets or outlets for gas and air, and they also hold the injection burners for heavy oil, tar, or natural gas, when used.
). Depending on the furnace size, the long front wall on the charging side usually has three to seven rectangular openings fitted with water-cooled doors. These are used for charging scrap and iron, adding flux and alloying agents, running off slag, conducting tests, and repairing the hearth refractory. On the opposite side of the furnace, at the back wall, is the taphole and a spout for tapping into one or two ladles. The two end walls are used as inlets or outlets for gas and air, and they also hold the injection burners for heavy oil, tar, or natural gas, when used.Above the hearth, an arched roof contains the flames and reflects the heat onto the melt. Since thermal exposure is intense here, the roof is made of high-grade chrome-magnesite refractory bricks suspended from a steel structure. Many furnaces have one to four retractable oxygen lances installed in the roof to increase the flame temperature and melting rate.
OHFs vary considerably in size, having been built for heats of 10 to 600 tons. The hearth of a 150-ton-capacity OHF is approximately 15 metres long and 5 metres wide. There are often up to a dozen furnaces in one shop, lined up end wall to end wall only a few metres apart with all front doors on one line and at the same level. This permits the charging of all furnaces by the same charging machine, crane, and rail system. Bulk materials, such as scrap, cold blast-furnace iron, ore, limestone, coke, and alloying agents, are charged through the furnace doors in small boxes of one- to two-cubic-metre capacity. The boxes are brought to the OHF on small railroad buggies, and a charging machine then moves one box after another through a door, turns them over, and dumps their contents onto the hearth.
The process
When starting a heat, the hearth is first covered by limestone flux, and scrap is charged on top of that. Charging a large furnace may require two to three hours and as many as 150 full charging boxes. The burners and oxygen lances are on during charging, so that most of the scrap has been melted by the end of the scrap charge. Afterward a special pouring spout is placed into one of the doors, and blast-furnace iron is slowly poured from an iron ladle into the melt. Composition of the metallic charge varies from 20 percent scrap and 80 percent blast-furnace iron to 100 percent scrap; a common proportion is 60 percent iron and 40 percent scrap.
Carbon in the poured iron reacts with the oxidized molten scrap and generates the carbon monoxide boil. This stirs the shallow (about 300 millimetres deep) bath and accomplishes a high heat transfer and a good mixing of the slag and metal. The carbon monoxide boil may last two to three hours, during which time carbon is oxidized and lowered, slag is flushed off through the doors, and the temperature is raised. Increasing heat causes the limestone charged beneath the scrap to calcine and release carbon dioxide, according to the following reaction:

This begins the lime boil, which has a beneficial stirring effect similar to that of the carbon monoxide boil. After about one hour, the calcined lime rises through the melt and is dissolved in the slag.
During the subsequent refining period, flux and alloys are added, and oxygen or carbon is injected to lower or raise the carbon content. When temperature and chemical composition are in the specified range, the furnace is tapped by blowing the taphole open with a small explosive charge. Tap-to-tap time is six to nine hours, often including one hour for inspection, cleaning, and hearth repair. After 200 to 300 heats, there is usually a three-day process of checker cleaning and more extensive repair work. The roof is usually replaced after about 1,000 heats, which shuts the furnace down for one week. The hearth, being made up anew after every heat, lasts many years.
Induction melting
Used by many specialty steelmaking shops and foundries, induction furnaces are cylindrical, open-topped, tiltable refractory crucibles (crucible process) with a water-cooled induction coil installed on the outside, around the side wall. The coil is powered by alternating current, which induces eddy currents in the metallic charge that generate heat. The refractory wall of the crucible is usually thin enough to achieve good penetration of the electromagnetic field into the charge.
Induction furnaces are used mainly for remelting and alloying and have very limited refining capabilities; in other words, they are not used for carbon, phosphorus, or sulfur removal. The slag is cold and not very active, and often there is no slag at all. However, the electromagnetic field stirs the melt well, and this is beneficial for alloying. Most furnaces' coils are powered by line frequency (i.e., 50 or 60 hertz), but there are also furnaces powered by medium frequency (e.g., up to 4,500 hertz), utilizing solid-state frequency converters. The electrical system always includes capacitor banks to compensate for the high inductance of the furnace coil. Efficiency of converting electric power into heat is about 75 percent, and power consumption is around 550 kilowatt-hours per ton of steel.
In commercial operation, a hot heel is often left in the furnace after tapping in order to decrease the thermal shock on the lining generated by the water-cooled coil. Smaller furnaces use prefabricated crucibles, but larger furnaces have a rammed—that is, compacted and dried—refractory mass as lining. Computer control is well utilized in this system, monitoring, for instance, the crucible lining thickness by the electrical performance of the furnace coil. The capacity of the furnace varies from a few kilograms to 50 tons.
Many induction furnaces are installed and operated in vacuum chambers. This is called vacuum induction melting, or VIM. When liquid steel is placed in a vacuum, removal of carbon, oxygen, and hydrogen takes place, generating a boil in the crucible. In many cases, the liquid steel is cast directly from the furnace into ingot molds that are placed inside the vacuum chamber.
Secondary steelmaking
The ladle
An open-topped cylindrical container made of heavy steel plates and lined with refractory, the ladle is used for holding and transporting liquid steel. Here all secondary metallurgical work takes place, including deslagging and reslagging, electrical heating, chemical heating or cooling with scrap, powder injection or wire feeding, and stirring with gas or with electromagnetic fields. The ladle receives liquid steel during tapping while sitting on a stand beneath the primary steelmaking furnace. It is moved by cranes, ladle cars, turntables, or turrets. A ladle turret has two liftable forks, usually 180° apart, that revolve around a tower, each fork capable of holding a ladle. Ladles have two heavy trunnions on each side for crane pickup. Support plates under each trunnion are used for setting the ladles onto stands or ladle cars.
The shell
The side wall of a ladle is slightly cone-shaped, with the larger diameter on top for easy removal of a skull—i.e., solidified steel and slag. A ladle capable of holding 200 tons of steel has an outside diameter of approximately four metres and is about five metres high. Inside the ladle there is usually a 60-millimetre-thick refractory safety lining next to the shell. The working lining, that part contacting the steel and slag, is 180 to 300 millimetres thick, depending on ladle size and location in the ladle. The lining thickness and type of brick in one ladle are often different to counteract increased wear at certain locations—for example, at the impact area of the tapping stream or at the slag line. This results in more equal wear on the ladle lining and an extended ladle service life.
Sometimes, fired clay bricks are used because they bloat—that is, they expand during heating and seal the joints between them. Their thermal shock resistance is high, but their resistance to slag corrosion is low, so that the working lining has to be replaced every 6 to 12 heats. Because ladle rebricking takes about eight hours, up to 12 ladles are sometimes in use in large steelmaking shops in order to assure availability. For ladle operations requiring longer holding times, higher-grade refractory linings are made of high alumina or magnesia bricks. These give greater slag resistance, but they do not bloat and are less resistant to thermal shock. For these reasons, they are kept hot at special preheating stations. Ladles that use these bricks have service lives of up to 80 heats, so that fewer ladles are required. Preheating also decreases the heat loss of liquid steel during tapping and holding.
Tapping
Except for very small ladles, which pour over the lip and a spout or through a teapot arrangement when tilted, most ladles have a funnel-shaped nozzle with a closing device installed in the bottom. Depending on ladle size, these nozzles have an orifice diameter of 15 to 100 millimetres and are made of high-grade refractory material. Often they are opened and closed by a vertical steel stopper rod, which is enclosed in refractory sleeves and partly immersed in the liquid steel. The head of the stopper rod closes the nozzle and is lifted a specific distance for controlling the flow rate; on top it is connected to a vertical slide that is either manually operated by a lever or remotely controlled from the crane pulpit.
Many shops use a slide-gate nozzle, which consists, in principle, of a fixed upper and a movable lower refractory plate. Both plates have holes that are adjusted relative to each other for closed, throttled, and full-open position. The lower plate is hydraulically shifted and is usually replaced after every heat. In a similar system, an old plate is pushed out by a new plate while pouring, and flow control is accomplished by using bottom plates with different orifice diameters. Having the entire flow-control system on the outside of the ladle and the inside of the ladle completely unrestricted is necessary for operating with long holding times and for certain steel treatments conducted in the ladle.
Stirring and storing
Ladles are often built with one or more permeable refractory bottom blocks and argon hookups for gas stirring. Ladles can also be placed against an electromagnetic stirring coil installed on a ladle car; in this case, their shells are made of a nonmagnetic alloy.
A number of shops use ladle lids to limit the liquid-steel heat loss. Lid-handling systems are normally mechanized, and removing, storing, and placing lids onto the ladles is done automatically.
Ladle metallurgy
The carrying out of metallurgical reactions in the ladle is a common practice in practically all steelmaking shops, because it is cost-efficient to operate the primary furnace as a high-speed melter and to adjust the final chemical composition and temperature of the steel after tapping. Also, certain metallurgical reactions, for reasons of equipment design and operation, are more efficiently performed in the ladle. The simplest form of steel treatment in the ladle takes place when the mixing effect of the tapping stream is used to add deoxidizers, slag formers, and small amounts of alloying agents. These materials are either placed into the ladle before tapping or are injected into the tapping stream.
Controlling temperature
Deoxidation reactions carried out in the ladle are exothermic and thus raise the temperature of the liquid steel, but the steel also loses heat by radiation from the top surface, by heating of the ladle lining, and by heat flux through the lining and shell. Temperature drops that take place when just holding the steel can range from 0.3° to 2° C per minute. (Small ladles, owing to their high surface-to-volume ratio, have a greater temperature loss than large ladles.) The rate of temperature drop then slows as the refractories become heated and a steady flow of heat prevails through the lining and slag layer.
 Tapping at the right temperature is necessary in order to meet critical temperature windows for teeming or casting operations. Heat losses during and after tap can usually be predicted by computer, using a process model that considers the temperature and configuration of the tap stream, the thermal condition of the ladle before tap, the thicknesses of the ladle lining and slag layer, the expected holding times and stirring conditions, and the thermal effects of alloying additions. Actual control over steel temperature can be achieved in a ladle furnace (LF). This is a small electric-arc furnace with an 8- to 25-megavolt-ampere transformer, three electrodes for arc heating, and the ladle acting as the furnace shell—as shown in A in thefigure-->
Tapping at the right temperature is necessary in order to meet critical temperature windows for teeming or casting operations. Heat losses during and after tap can usually be predicted by computer, using a process model that considers the temperature and configuration of the tap stream, the thermal condition of the ladle before tap, the thicknesses of the ladle lining and slag layer, the expected holding times and stirring conditions, and the thermal effects of alloying additions. Actual control over steel temperature can be achieved in a ladle furnace (LF). This is a small electric-arc furnace with an 8- to 25-megavolt-ampere transformer, three electrodes for arc heating, and the ladle acting as the furnace shell—as shown in A in thefigure--> . Argon or electromagnetic stirring is applied for better heat transfer. Most LFs can raise the temperature of the steel by 4° C per minute, and several shops accomplish an increase of 4° to 6° C by inducing a strong exothermic chemical reaction (for instance, by feeding aluminum and injecting oxygen) at the stirring station. Subsequent argon stirring removes most of the alumina inclusions formed by this process. Both heating technologies permit long holding times of full ladles and improve the continuous caster operation.
. Argon or electromagnetic stirring is applied for better heat transfer. Most LFs can raise the temperature of the steel by 4° C per minute, and several shops accomplish an increase of 4° to 6° C by inducing a strong exothermic chemical reaction (for instance, by feeding aluminum and injecting oxygen) at the stirring station. Subsequent argon stirring removes most of the alumina inclusions formed by this process. Both heating technologies permit long holding times of full ladles and improve the continuous caster operation.Slag removal
Keeping furnace slags on the molten steel too long can result in a reversion of elements such as phosphorus back into the steel. To avoid this, slag can be removed at slag-skimming stations, where the ladle is tilted forward and a rake scrapes the slag into a slag pot parked beneath the ladle. Some shops use a vacuum system, which sucks the slag off the liquid steel and granulates it instantaneously. In either case, after slag removal the steel is covered with slag formers or an insulating layer to minimize heat loss and reoxidation. Special equipment is used to quickly place a blanket of material on the steel surface.
Stirring and injecting
In most continuous casting operations, it is necessary to maintain minimal fluctuation in steel temperature, and this requires the use of a ladle stirring station to establish a uniform temperature and chemical composition throughout the ladle. The steel can be stirred by argon injected through a refractory-lined lance or through a permeable refractory block in the bottom of the ladle, or it can be stirred by an electromagnetic coil.
Additions are usually made at the stirring station by a wire feeder, which runs a heavy wire at controlled speed through a refractory-covered lance and into the steel. Aluminum wire is often used for trimming; other materials, such as calcium-silicon, zirconium, and rare-earth metals, are often enclosed in thin steel tubes and are fed by the same machines. The wires and filled tubes are normally shipped to steel plants in large coils, but there are also machines that fill the tubes with the appropriate materials on-site.
Another widely used treatment is powder injection. Powdered metal is fluidized by argon in a pressure vessel and injected by a refractory-lined lance deep into the liquid steel. Because powder has a large contact surface area, it reacts quickly with the steel. Deep injection is beneficial when adding materials such as calcium or magnesium, which evaporate at steelmaking temperature, because ferrostatic pressure suppresses the evaporation of these metals for some time. Powders are shipped to the shop in sealed containers or in special tank cars topped with inert gas.
Desulfurizing
Many powder-injection stations are used for desulfurization. One effective desulfurizer is a calcium-silicon alloy containing 30 percent calcium. Metallic calcium desulfurizes by forming the very stable compound calcium sulfide (CaS), and it is alloyed with silicon because pure calcium reacts instantaneously with water and is therefore difficult to handle. Injecting four kilograms of calcium-silicon per ton of steel can remove approximately three-quarters of the sulfur, so that the sulfur content will drop, for example, from 0.016 to 0.004 percent. For steel grades that do not permit silicon additions, a magnesium-lime mixture is used. Magnesium is a good desulfurizer, and it also acts as a deoxidizer by combining locally with dissolved oxygen. This makes it possible for the lime to desulfurize the steel according to the following reaction:

Like magnesium, lime has a double function, because it helps to prevent the very low-melting magnesium powder from melting inside the lance.
Adding calcium accomplishes another important function. Sulfur is normally present in solidified steel in the form of manganese sulfide inclusions, which are soft at hot-rolling temperatures and are rolled into long strings or platelets. This results in poor physical properties of the steel in directions perpendicular to that of the rolling. The addition of calcium improves these properties by forming strong inclusions, containing mainly calcium sulfide, that are not plastic at hot-rolling temperatures. This phenomenon, called inclusion shape control, can also be achieved by small additions of zirconium or rare earth.
Vacuum treatment
Exposing steel to vacuum conditions has a profound effect on all metallurgical reactions involving gases. First, it lowers the level of gases dissolved in liquid steel. Hydrogen, for example, is readily removed in a vacuum to less than two parts per million. Nitrogen is not as mobile in liquid steel as hydrogen, so that only 15 to 30 percent is typically removed during a 20-minute vacuum treatment.
Another important process is vacuum decarburization and deoxidation. In theory, oxygen and carbon, when dissolved in steel, react to form carbon monoxide until they reach equilibrium at the following relationship:

This means that, under vacuum conditions (when there are only small amounts of carbon monoxide in the surrounding gas and therefore little carbon monoxide pressure), carbon and oxygen will react vigorously until they reach equilibrium at very low levels. For instance, liquid steel at 1 atmosphere pressure may contain 0.043 percent carbon and 0.058 percent oxygen, but, if the pressure is lowered to 0.1 atmosphere, the two elements will react until they reach equilibrium at 0.014 percent carbon and 0.018 percent oxygen. Under a pressure as low as 0.01 atmosphere, equilibrium will be reached at 0.004 percent carbon and 0.006 percent oxygen. In practical operation, the obtainable levels of carbon and oxygen are far above equilibrium conditions, because the movement of carbon and oxygen atoms in liquid steel is time-consuming and treatment time is limited. In addition, the steel is continuously reoxidized by multiple sources of oxygen. Nevertheless, it is common practice to produce ultralow-carbon steel, containing less than 0.003 percent carbon, in 20 minutes at a vacuum treatment station under pressure of one torr. (In vacuum technology, pressures are often expressed in torr, which is equivalent to the pressure of a column of one millimetre of mercury. One atmosphere equals 760 torr.)
 There are several types of vacuum treatment, their use depending on steel grade and required production rates. In the tank degasser (shown in B in thefigure-->
There are several types of vacuum treatment, their use depending on steel grade and required production rates. In the tank degasser (shown in B in thefigure--> ), the ladle is placed in an open-top vacuum tank, which is connected to vacuum pumps. The vacuum pumping system often consists of two or three mechanical pumps, which lower the pressure to about 0.1 atmosphere, and four or five stage steam ejectors, which bring the pressure to under 1 torr, or 0.0013 atmosphere. Practical treatment time is 20 to 30 minutes. The ladles used in tank degassing stations are large and, when filled with steel, retain about one metre of freeboard in order to contain the melt during a vigorous boil.
), the ladle is placed in an open-top vacuum tank, which is connected to vacuum pumps. The vacuum pumping system often consists of two or three mechanical pumps, which lower the pressure to about 0.1 atmosphere, and four or five stage steam ejectors, which bring the pressure to under 1 torr, or 0.0013 atmosphere. Practical treatment time is 20 to 30 minutes. The ladles used in tank degassing stations are large and, when filled with steel, retain about one metre of freeboard in order to contain the melt during a vigorous boil.A modification of the tank degassers is the vacuum oxygen decarburizer (VOD), which has an oxygen lance in the centre of the tank lid to enhance carbon removal under vacuum. The VOD is often used to lower the carbon content of high-alloy steels without also overoxidizing such oxidizable alloying elements as chromium. This is possible because, in the pressure-dependent carbon-oxygen reaction outlined above, oxygen reacts with carbon before it combines with chromium. The VOD is often used in the production of stainless steels.
There are also tank degassers that have electrodes installed like a ladle furnace, thus permitting arc heating under vacuum. This process is called vacuum arc degassing, or VAD.
 For higher production rates (e.g., 25 ladles treated per day) and large ladles (e.g., 200 tons), a recirculation degasser is used, as shown in C in thefigure-->
For higher production rates (e.g., 25 ladles treated per day) and large ladles (e.g., 200 tons), a recirculation degasser is used, as shown in C in thefigure--> . This has two refractory-lined snorkels that are part of a high, cylindrical, refractory-lined vacuum vessel and are immersed in the steel. As the system is evacuated, atmospheric pressure pushes the liquid steel through the snorkels and up into the vessel. One atmosphere lifts liquid steel about 1.3 metres. Injecting argon into one of the snorkels then circulates the steel through the vessel, continuously exposing a portion of the steel to the vacuum. Recirculation facilities are often very elaborate, using fast vessel-exchange systems or even two operating vessels at one station to achieve high production rates. Some units also inject oxygen during vacuum treatment, through either the side or the top of the vessel. This is done to speed up decarburization or, by simultaneously adding aluminum, to increase the steel temperature. Some shops apply a similar system but use a vacuum vessel with only one snorkel. Here, a portion of the steel in the ladle flows in and out of the vacuum vessel and is exposed to the vacuum by a continuous raising and lowering of either the vessel or the ladle.
. This has two refractory-lined snorkels that are part of a high, cylindrical, refractory-lined vacuum vessel and are immersed in the steel. As the system is evacuated, atmospheric pressure pushes the liquid steel through the snorkels and up into the vessel. One atmosphere lifts liquid steel about 1.3 metres. Injecting argon into one of the snorkels then circulates the steel through the vessel, continuously exposing a portion of the steel to the vacuum. Recirculation facilities are often very elaborate, using fast vessel-exchange systems or even two operating vessels at one station to achieve high production rates. Some units also inject oxygen during vacuum treatment, through either the side or the top of the vessel. This is done to speed up decarburization or, by simultaneously adding aluminum, to increase the steel temperature. Some shops apply a similar system but use a vacuum vessel with only one snorkel. Here, a portion of the steel in the ladle flows in and out of the vacuum vessel and is exposed to the vacuum by a continuous raising and lowering of either the vessel or the ladle.Argon-oxygen decarburization
In the production of stainless steel and other high-alloy grades that contain highly oxidizable elements such as chromium, lowering the levels of carbon by regular oxygen injection has the undesirable consequence of oxidizing the alloying elements as well. The argon-oxygen decarburization (AOD) process alleviates this problem by diluting the injected oxygen with argon. This lowers the partial pressure of oxygen and carbon monoxide, so that, based on the pressure-dependent equilibrium relationship %C × %O = 0.0025 × CO pressure, the oxygen prefers to combine with carbon and oxidizes only a small amount of alloy.
The converter
The AOD process is carried out in a refractory-lined converter similar to the BOF but with two to six argon-oxygen tuyeres installed in the lower side wall. The tuyeres consist of two concentric steel tubes, with the inert gas flowing in the outer annulus and oxygen in the inner tube. The converter has tilting and emission-control equipment similar to that of the BOF; the lining is also basic, but it lasts only 50 to 100 heats because of the long refining time and the high temperature of more than 1,700° C (3,100° F) that is necessary for improving the chromium yield. Most shops have three converter shells and one trunnion ring at a blowing station, rotating them between operation, relining, and preheating.
The process
When making austenitic stainless steel, the AOD converter is charged with liquid high-carbon chromium-nickel steel that has been melted in a regular EAF and may contain 1.5 percent carbon, 19 percent chromium, and 10 percent nickel. The blow starts with a high-oxygen gas mixture of, for instance, 80 percent oxygen and 20 percent argon, because there is still plenty of carbon in the steel with which oxygen prefers to combine. As the carbon level drops, the gas mixture is gradually changed into one rich in argon; this may end with a blowing gas of 20 percent oxygen and 80 percent argon. After a blowing time of about one hour, the final carbon content is on the order of 0.015 percent, and only about 2 percent chromium has been lost. The steel is then deoxidized by ferrochrome silicon and desulfurized with burnt lime. Argon is also blown during this end phase for better mixing and removal of hydrogen and nitrogen.
The tap-to-tap time is about two hours, and consumption of oxygen and argon is about 25 and 20 cubic metres, respectively, per ton of steel. To minimize cost, argon is sometimes replaced by nitrogen or compressed air at the beginning of the blow. AOD converters with capacities up to 160 tons are in operation.
casting of steel
ingot pouring
The simplest way to solidify liquid steel is to pour it into heavy, thick-walled iron ingot molds, which stand on stout iron plates called stools.
Solidification processes

 During and after pouring, the walls and bottom of the mold extract heat from the melt, and a solid shell forms, growing approximately with the square root of time multiplied by a constant. The value of the constant depends on the heat flux between the already solidified shell and the cooling media surrounding it and is actually equivalent to the solidified shell's thickness after one minute—namely, about 20 millimetres when solidifying steel. Accordingly, the ingot shell is about 40 millimetres thick after four minutes and 60 millimetres after nine minutes. As the shell thickens, the level of the liquid melt in the centre of the mold drops, because solidified steel has a higher density than liquid steel—i.e., 7.86 versus 7.06 grams per cubic centimetre (4.5 versus 4.1 ounces per cubic inch). This creates a cavity on top of the ingot, as shown in A in the figure-->
During and after pouring, the walls and bottom of the mold extract heat from the melt, and a solid shell forms, growing approximately with the square root of time multiplied by a constant. The value of the constant depends on the heat flux between the already solidified shell and the cooling media surrounding it and is actually equivalent to the solidified shell's thickness after one minute—namely, about 20 millimetres when solidifying steel. Accordingly, the ingot shell is about 40 millimetres thick after four minutes and 60 millimetres after nine minutes. As the shell thickens, the level of the liquid melt in the centre of the mold drops, because solidified steel has a higher density than liquid steel—i.e., 7.86 versus 7.06 grams per cubic centimetre (4.5 versus 4.1 ounces per cubic inch). This creates a cavity on top of the ingot, as shown in A in the figure--> by a schematic presentation of solidifying layers. Since an open cavity oxidizes, it does not weld during hot rolling and must be cut off, resulting in a loss of steel. The cavity can be made shallower by keeping the top of the ingot hot and liquid longer. This is done by inserting insulating refractory heads (as shown in C in the figure-->
by a schematic presentation of solidifying layers. Since an open cavity oxidizes, it does not weld during hot rolling and must be cut off, resulting in a loss of steel. The cavity can be made shallower by keeping the top of the ingot hot and liquid longer. This is done by inserting insulating refractory heads (as shown in C in the figure--> ) and by adding exothermic powders; more liquid steel can also be added after a good-sized shell has formed.
) and by adding exothermic powders; more liquid steel can also be added after a good-sized shell has formed.
 The solidification pattern described above can be observed in well-deoxidized steel, which shows no evolution of gas as it solidifies. For this reason, it is called a killed steel. A different solidification pattern is applied to certain other steels to which fewer deoxidizers have been added. These contain a controlled amount of dissolved oxygen, which, during solidification, reacts with carbon and generates a mild carbon monoxide boil. The rising carbon monoxide bubbles stir the melt, lift inclusions, and cause the formation of a very clean shell about 50 millimetres thick, called the rim. After the rim has formed, a cooling plate is placed on top of the ingot, freezing a layer of liquid steel and trapping the gas bubbles inside the solidifying ingot, as shown in B in the figure-->
The solidification pattern described above can be observed in well-deoxidized steel, which shows no evolution of gas as it solidifies. For this reason, it is called a killed steel. A different solidification pattern is applied to certain other steels to which fewer deoxidizers have been added. These contain a controlled amount of dissolved oxygen, which, during solidification, reacts with carbon and generates a mild carbon monoxide boil. The rising carbon monoxide bubbles stir the melt, lift inclusions, and cause the formation of a very clean shell about 50 millimetres thick, called the rim. After the rim has formed, a cooling plate is placed on top of the ingot, freezing a layer of liquid steel and trapping the gas bubbles inside the solidifying ingot, as shown in B in the figure--> . This ingot has no open cavity, but there are many blowholes in the centre that normally weld together during hot-rolling. Low-carbon steel, because of its higher dissolved oxygen content, is often cast this way and is called rimmed steel. Normally, rimmed steel is cast into a big-end-down mold, as shown in B in the figure-->
. This ingot has no open cavity, but there are many blowholes in the centre that normally weld together during hot-rolling. Low-carbon steel, because of its higher dissolved oxygen content, is often cast this way and is called rimmed steel. Normally, rimmed steel is cast into a big-end-down mold, as shown in B in the figure--> , for easier mold stripping and ingot handling.
, for easier mold stripping and ingot handling.An important characteristic of all solidification processes is segregation. This takes place when crystals grow in a multicomponent melt, because crystals are always purer than the liquid melt from which they solidify. Therefore, as steel solidifies, the levels of carbon, phosphorus, and sulfur grow in the remaining liquid, resulting in an enrichment of these elements in the centre of the ingot. Segregation can be minimized by keeping segregating elements at low levels or by solidifying at a fast rate—i.e., by not providing the time for separation. It is also impaired by stirring the melt.
Pouring procedures
 The layouts of pouring pits differ greatly, depending on the type of steel produced and the rate of production. In top pouring conducted in high-tonnage shops, a row of perhaps 20 molds is lined up in buggies on a railroad track in front of a pouring platform (see figure-->
The layouts of pouring pits differ greatly, depending on the type of steel produced and the rate of production. In top pouring conducted in high-tonnage shops, a row of perhaps 20 molds is lined up in buggies on a railroad track in front of a pouring platform (see figure--> ). A crane brings the ladle to the platform and holds it while the operator fills one mold after another. After standing for a specified time, the molds are pulled out of the teeming aisle and into a stripper building, where they are lifted from the ingots. In a different procedure, called bottom pouring, as many as six ingot molds stand on a single large and thick bottom plate with several pipelike refractory runners installed on its top surface. These runners connect the molds to a refractory-lined, funnel-shaped feeder tube, which receives liquid steel from the ladle and directs it to the molds, filling them simultaneously from the bottom. Bottom pouring avoids the splashing from the ladle stream that is experienced during top pouring. The system is often completely mechanized, with the bottom plates movable on wide transfer tracks and prepared for the next use away from the pouring aisle.
). A crane brings the ladle to the platform and holds it while the operator fills one mold after another. After standing for a specified time, the molds are pulled out of the teeming aisle and into a stripper building, where they are lifted from the ingots. In a different procedure, called bottom pouring, as many as six ingot molds stand on a single large and thick bottom plate with several pipelike refractory runners installed on its top surface. These runners connect the molds to a refractory-lined, funnel-shaped feeder tube, which receives liquid steel from the ladle and directs it to the molds, filling them simultaneously from the bottom. Bottom pouring avoids the splashing from the ladle stream that is experienced during top pouring. The system is often completely mechanized, with the bottom plates movable on wide transfer tracks and prepared for the next use away from the pouring aisle. Iron molds are cleaned and repaired in a mold yard. Depending on practice, they are replaced after 40 to 70 pours. Most specialty steel shops pour their alloy grades in big-end-up molds and use hot tops, as shown in C in the figure-->
Iron molds are cleaned and repaired in a mold yard. Depending on practice, they are replaced after 40 to 70 pours. Most specialty steel shops pour their alloy grades in big-end-up molds and use hot tops, as shown in C in the figure--> , in order to minimize the size of the cavity and consequent steel loss. All large ingots—for instance, 200-ton ingots intended for forgings—are also poured this way.
, in order to minimize the size of the cavity and consequent steel loss. All large ingots—for instance, 200-ton ingots intended for forgings—are also poured this way.Continuous casting
About 55 percent of the world's liquid steel production is solidified in continuous casting processes, the most widely used of which feeds liquid steel continuously into a short, water-cooled vertical copper mold and, at the same time, continuously withdraws the frozen shell, including the liquid steel it contains.
Tundish, mold, and secondary zone
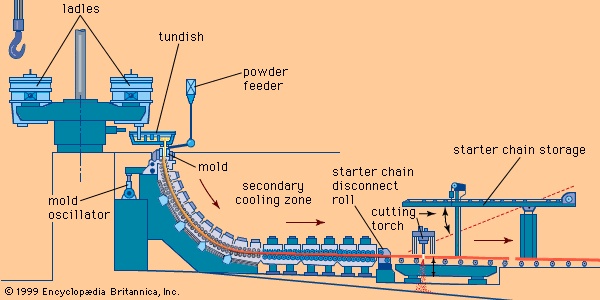 The key control parameter of continuous casting is matching the flow of liquid steel into the mold with the withdrawal speed of the strand out of the mold. The control of flow rates is accomplished by the tundish, a small, refractory-lined distributer that is placed over the mold and that receives steel from the furnace ladle (see figure-->
The key control parameter of continuous casting is matching the flow of liquid steel into the mold with the withdrawal speed of the strand out of the mold. The control of flow rates is accomplished by the tundish, a small, refractory-lined distributer that is placed over the mold and that receives steel from the furnace ladle (see figure--> ). Withdrawal speed is controlled by driven rolls, which contact the strand at a point where it has already developed a thick, solidified shell.
). Withdrawal speed is controlled by driven rolls, which contact the strand at a point where it has already developed a thick, solidified shell.Feeding of the caster mold from the tundish is controlled by a stopper rod or a sliding gate similar to the equipment used in ladles (see above Secondary steelmaking: The ladle: Tapping (steel)). The liquid steel in the tundish must be within a specific temperature “window”—a range just above its liquidus that is determined by the steel's grade; in addition, measures are always taken to keep air away from the steel in order to minimize reoxidation. Shielding can be accomplished by pouring steel through refractory tubes that are immersed in the steel or through wide sleeves that are pressurized with argon. The tundish itself is covered with a lid and is often also topped with argon. Both ladle and tundish sit on a turret or transfer car to permit a quick exchange.
The mold is made of copper because of the high heat conductivity of that metal. It is heavily water-cooled and oscillates up and down to avoid sticking of the solidified shell to its walls. In addition, the mold wall is lubricated by oil or slag, which is maintained on the steel meniscus and flows down into the gap between mold and strand. The slag layer, when used, is formed by the continuous addition of casting powder. Besides providing lubrication, it keeps air away from the liquid steel, acts as a heat barrier, and absorbs inclusions.
Many continuous casters contain sensors in the mold for automatically synchronizing the flow of liquid steel into the mold with the strand withdrawal speed. As it exits the mold, the strand has a shell thickness of only about 10 millimetres and is immediately water-cooled by spray nozzles. The strength and soundness of the shell at this location determine the maximum casting speed, because rupturing it would result in a breakout of liquid steel and damage to the caster. On its way down, the strand is supported by many rolls to avoid a bulging of the shell by the ferrostatic pressure of the liquid steel it contains. As the shell thickness increases toward the end of this so-called secondary cooling zone, the supporting rolls grow larger and are spaced farther apart. The secondary zone is often also called the metallurgical length, because this is where the strand solidifies and the cast structure develops. Depending on the strand's cross section and the casting speed, it can be 10 to 40 metres long. The flow of water to the many nozzles in the various sections is often computer-controlled and automatically adjusted as casting conditions change.
After the strand passes through the last pair of support rolls, it enters the run-out table and is cut, while moving, by one or two oxyacetylene torches.
Design principles
Continuous casters in commercial operation are built according to different design principles. For some steels and solidification patterns, all components are arranged in a vertical line—a straight mold, a straight secondary cooling zone, and vertical strand cutting. Other casters also have a straight mold and a vertical secondary cooling zone, but they bend the strand on its way down, after it has solidified, into a horizontal direction and cut it on a run-out table. (In spite of the horizontal turn, even this design requires a high building and a long ladle lift.)
 The majority of continuous casters have a curved mold, a curved secondary cooling zone, and a series of straightening rolls before the horizontal run-out table. Everything down to the straightener is on one radius or on several matching radii. This design results in a low casting machine, as shown in the figure-->
The majority of continuous casters have a curved mold, a curved secondary cooling zone, and a series of straightening rolls before the horizontal run-out table. Everything down to the straightener is on one radius or on several matching radii. This design results in a low casting machine, as shown in the figure--> .
.Billet, bloom, beam, and slab
Different design principles are used for casting strands of different cross sections. Billet casters solidify 80- to 175-millimetre squares or rounds, bloom casters solidify sections of 300 by 400 millimetres, and beam blank casters produce large, dog-bone-like sections that are directly fed into an I-beam or H-beam rolling mill. Huge slab casters solidify sections up to 250 millimetres thick and 2,600 millimetres wide at production rates of up to three million tons per year.
In order to match the quantity of steel produced in a heat with the solidification capacity of a mold for a certain strand section, it is often necessary to use a multistrand caster. Some billet casters have six molds in one line next to one another, and all are fed from the same tundish.
casting procedures
To begin casting, a starter head matching the inside dimension of the mold and connected to a starter chain is moved up into the mold. The starter chain has dimensions similar to the strand to be cast and is long enough to be moved up and down by the driven rolls. When liquid steel fills the mold, it freezes to the caster head, which is immediately withdrawn. The chain in front of the solidifying strand moves through the secondary cooling zone, and, after the head has cleared the last support roll, it is disconnected from the strand by an upward-moving push-out roll. The chain is then pulled by a winch onto a support cradle, lifted from the table, and stored for reuse. At the end of casting, when the tundish is almost empty, the flow of steel to the mold is discontinued, and the strand is stopped and, after solidifying, completely withdrawn. For the next cast, the starter chain, with the head in front, is moved again by the driven rolls into the secondary cooling zone and mold.
Casting of one ladle takes 45 to 90 minutes, depending on heat size, steel grade, caster layout, and casting conditions. Turning the caster around—that is, preparing it for the next cast—is usually accomplished in a half hour, but it takes longer when the mold is changed for casting a different section. Slab casters often use molds with movable side plates, thus permitting a fast change of width during caster turnaround or even during casting. Such devices, together with fast exchange systems for casting tubes, tundishes, and ladles, permit sequential heats to be cast without stopping the caster—sometimes for several days. Starting and stopping a caster causes a few metres of steel on both ends of the strand to fall below the specified properties, thereby lowering the steel-to-strand yield. In sequential casting, on the other hand, the yield from liquid steel to acceptable strand approaches 100 percent, compared with perhaps 93 percent when turning the caster around after each ladle or to 86 percent in an ingot-casting operation that uses a blooming or slabbing mill to roll a slab or bloom of the same size. The benefits are substantial because much less raw material, liquid steel, and energy are needed to make the same tonnage of cast product.
Metallurgical quality is often enhanced by computer control over some or all systems of the caster. Casting conditions are often further improved by electrical tundish heating to adjust steel temperature, by electromagnetic stirring coils around the strand to decrease segregation, by in-line rolling to compact the centre just before it solidifies, and, most important, by well-designed inspection systems to check the liquid steel and the hot strand during casting. Such systems provide a high level of quality assurance, making it possible to charge the cut strand hot into a reheat furnace or, with only a little reheating of the edges, directly into a hot-rolling mill. This not only minimizes reheating but eliminates cooling, cold inspection, scarfing or grinding, and storage. Plants that integrate a continuous caster with a hot-rolling mill often need only 90 minutes to convert liquid steel into a hot-rolled product.
Variations
Some plants have been built specifically for direct rolling. One example is a thin-slab caster that casts strands 50 millimetres thick and 1,250 millimetres wide at speeds of about five metres per minute. After the strand is cut on the run-out table, the slabs are directly heated in-line in a long tunnel furnace or by induction coils and then fed, also in-line, directly into the finishing train of a hot-strip mill. With everything in one continuous line, operating and maintenance systems must be kept at the highest level.
Another special continuous process is the rotary casting of rounds, mainly for seamless tubes. A rotary caster is similar to a straight-mold vertical caster, except that the round mold, the strand, and the withdrawal system revolve at about 75 rotations per minute. This creates a centrifugal force within the strand and results in a cleaner cast and better contact between strand and mold. Still another variation is the casting of rounds in a horizontal caster. This entirely different system employs a large tundish with a horizontal nozzle in its side wall that extends directly into a water-cooled horizontal mold. The strand oscillates and is pulled out of the mold in small increments each time a new shell has formed at the mold entrance. Everything is located on one level, so that there are no high ladle lifts. Ferrostatic pressure in the strand is also very low, but segregation tendencies caused by gravitational forces require more careful preparation of the liquid steel.
There have been, and still are, many continuous-casting concepts tested in laboratories, pilot plants, and trial operations. Examples include single- or dual-roll strip casters, which cast strip directly from liquid steel, and belt casters for thin-slab production. There have also been hundreds of patents issued on continuous casting, all with the goal of making the process more cost-efficient, improving metallurgical control, and casting as close to the final product shape as possible.
Special solidification (ingot) processes
For the manufacture of special products, refining and solidification processes are often combined.
Vacuum ingot pouring
Vacuum ingot pouring is often employed to produce very large ingots that are subsequently processed, in expensive forging and machining operations, into such products as rotors for power generators. In this process, an ingot mold is placed inside a cylindrical tank that is connected to vacuum pumps. The tank is closed by a lid, and a small, stopper-operated ladle having a capacity of about 25 tons of liquid steel is set on top of the lid. The nozzle of this so-called pony ladle is sealed by an aluminum disk, the tank is evacuated, and the furnace ladle starts pouring steel into the pony ladle. When the ferrostatic pressure reaches a certain point, the stopper is opened, the aluminum plate burns through, and the stream of liquid steel is degassed before it fills the mold for solidification. Pouring under vacuum lowers the hydrogen content, an important matter for large ingots.
Vacuum arc remelting (VAR)
In this process, employed for casting steels that contain easily oxidized alloying elements, a consumable electrode made of forged steel or of compacted powder or sponge is continuously melted by an arc under vacuum. At the same time, the shallow molten pool underneath the electrode is continuously solidified in a water-cooled, normally round copper mold. As the mold is filled, the electrode moves up. The melting current, in flowing between the electrode and the mold, passes through the arc, liquid pool, and solidified strand. Melting under high vacuum lowers the levels of dissolved oxygen, oxide inclusions, hydrogen, nitrogen, and elements having a high vapour pressure, such as lead, manganese, and tin. In addition, the shallow pool results in a directional solidification, with the crystals growing parallel to the axes of the ingot; this greatly improves the subsequent hot-forming operation. There is no segregation and no cavity. Ingots weighing up to 50 tons and measuring 1.5 metres in diameter have been cast with this method.
Electroslag remelting (ESR)
In this process, there is a slowly melting consumable electrode and a water-cooled mold for solidification, as in vacuum arc remelting, but the melting is conducted under normal atmosphere and is accomplished by a thick, superheated layer of slag on top of the shallow metal pool. This slag is resistance-heated by the high electrical current passing from the electrode to the mold, and it also desulfurizes the molten steel drops as they pass through on their way from the electrode to the liquid pool. Solidification patterns are similar to those in vacuum arc remelting. The ingot surface is very clean, owing to the presence of a slag layer between the ingot and mold, and does not need surface conditioning. Some electroslag installations cast ingots heavier than 200 tons.
Steel foundry (founding)
Foundries that cast steel into commercial products mainly employ coreless induction furnaces or electric-arc furnaces for melting scrap. Scrap quality is normally high because a large portion of return scrap is used in the form of gates and risers left over from previous casting operations. Since it is often not necessary to refine scrap—that is, to lower the sulfur and phosphorus content—an acid process can be applied using a high-silica slag that may contain 60 percent silica, 10 percent lime, 10 percent manganese oxide, and 15 percent iron oxide. This permits the furnaces to run with a cheaper acid lining.
Tapping temperatures are usually higher than for ingot pouring or continuous casting in order to have a liquid steel with good fluidity that fills the thin parts of a casting. Molding is similar to that in gray-iron foundries, but a more heat-resistant mold material is necessary because of the higher temperatures. Solidifying steel castings normally show a higher linear shrinkage (1.5 percent) than gray iron castings, which shrink about 1 percent. Small parts are cast in greensand molds, but larger parts are made in stronger dry-sand molds.
Forming of steel
Principles
Forming processes convert solidified steel into products useful for the fabricating and construction industries. The objectives are to obtain a desired shape, to improve cast steel's physical properties (which are not suitable for most applications), and to produce a surface suitable for a specific use. During plastic forming, the large crystals in cast steel are converted into many small, long crystals, transforming the usually brittle cast into a ductile and tough steel. In order to accomplish this, it is often necessary to reduce the cross section of a cast structure to one-eighth or even less of its original.
The major forming processes are carried out hot, at about 1,200° C (2,200° F), because of steel's low resistance to plastic deformation at this temperature. This requires the use of reheating furnaces of different designs. Cold forming is often applied as a secondary process for making special steel products such as sheet or wire.
 There are a number of steel-forming processes—including forging, pressing, piercing, drawing, and extruding—but by far the most important one is rolling. In this process, the rolls, working always in pairs, are driven in opposite directions with the same peripheral velocity and are held at a specific distance from each other by heavy bearings and mill housings. The steel workpiece is pulled by friction into the roll gap, which is smaller than the cross section of the workpiece, so that both rolls exert a pressure and continuously form the piece until it leaves the roll gap with a smaller section and increased length. As shown in the figure-->
There are a number of steel-forming processes—including forging, pressing, piercing, drawing, and extruding—but by far the most important one is rolling. In this process, the rolls, working always in pairs, are driven in opposite directions with the same peripheral velocity and are held at a specific distance from each other by heavy bearings and mill housings. The steel workpiece is pulled by friction into the roll gap, which is smaller than the cross section of the workpiece, so that both rolls exert a pressure and continuously form the piece until it leaves the roll gap with a smaller section and increased length. As shown in the figure--> , the reduction in cross section is calculated by subtracting the out-section (S2) from the in-section (S1) and then dividing by S1. Assuming the workpiece maintains its original volume as it is formed, the elongation (L2) divided by the original length (L1) equals S1 divided by S2. When rolling flat products, there is not much change in width, so that the thickness alone can be used to calculate reduction.
, the reduction in cross section is calculated by subtracting the out-section (S2) from the in-section (S1) and then dividing by S1. Assuming the workpiece maintains its original volume as it is formed, the elongation (L2) divided by the original length (L1) equals S1 divided by S2. When rolling flat products, there is not much change in width, so that the thickness alone can be used to calculate reduction.

 The basic principles of a rolling-mill design are shown in B in the figure-->
The basic principles of a rolling-mill design are shown in B in the figure--> . Two heavy bearings mounted on each side of a roll sit in chocks, which slide in a mill housing for adjusting the roll gap with a screw. The two housings are connected to each other and to the foundation, and the complete assembly is called a roll stand. There are also compact rolling units (C in the figure-->
. Two heavy bearings mounted on each side of a roll sit in chocks, which slide in a mill housing for adjusting the roll gap with a screw. The two housings are connected to each other and to the foundation, and the complete assembly is called a roll stand. There are also compact rolling units (C in the figure--> ), which do not have housings; often used in the tandem rolling of long products, they can be exchanged quickly for repair or for a change in the rolling program. Rolls are driven through spindles and couplings, either directly or via a gear, by one or several electric motors. Depending on the product rolled, there are stands that have two, three, four, and more rolls; accordingly, they are given the names two-high, three-high, four-high, six-high, cluster mill, and planetary mill (schematically shown in the figure-->
), which do not have housings; often used in the tandem rolling of long products, they can be exchanged quickly for repair or for a change in the rolling program. Rolls are driven through spindles and couplings, either directly or via a gear, by one or several electric motors. Depending on the product rolled, there are stands that have two, three, four, and more rolls; accordingly, they are given the names two-high, three-high, four-high, six-high, cluster mill, and planetary mill (schematically shown in the figure--> ). For rolling strip, heavy backup rolls support the smaller work rolls, because thin rolls form flat material better than do large-diameter rolls.
). For rolling strip, heavy backup rolls support the smaller work rolls, because thin rolls form flat material better than do large-diameter rolls.
 In a rolling shop, stands are arranged according to three layout principles. One is called the open train (G in the figure-->
In a rolling shop, stands are arranged according to three layout principles. One is called the open train (G in the figure--> ), in which the stands are arranged side by side, often driven by the same motor and linked by spindles. This arrangement is applied only to the rolling of long products, with guides or cross-transfers being used to move the workpiece from stand to stand. A tandem mill arrangement (H in the figure-->
), in which the stands are arranged side by side, often driven by the same motor and linked by spindles. This arrangement is applied only to the rolling of long products, with guides or cross-transfers being used to move the workpiece from stand to stand. A tandem mill arrangement (H in the figure--> ) has one stand behind the other and is used for high-production rolling of almost all products. This continuous arrangement requires the construction of long rolling trains and buildings, but layouts can be shortened by a so-called semicontinuous mill, in which the workpiece is passed back and forth through a reversing mill before being sent through the rest of the line. When open-train and tandem arrangements are combined for rolling long products in more compact layouts, it is called a cross-country mill.
) has one stand behind the other and is used for high-production rolling of almost all products. This continuous arrangement requires the construction of long rolling trains and buildings, but layouts can be shortened by a so-called semicontinuous mill, in which the workpiece is passed back and forth through a reversing mill before being sent through the rest of the line. When open-train and tandem arrangements are combined for rolling long products in more compact layouts, it is called a cross-country mill.Slabs and blooms
Cast ingots, sometimes still hot, arrive at slabbing and blooming mills on railroad cars and are charged upright by a special crane into under-floor soaking pits. These are gas-fired rectangular chambers, about 5 metres deep, in which four to eight ingots are simultaneously heated to about 1,250° C (2,300° F). An ingot used for conversion into a slab can be 1.5 metres wide, 0.8 metre thick, and 2.5 metres high and can weigh 23 tons. The soaking pits are highly computerized for scheduling, firing rates, heating times (which can last 8 to 18 hours), and rolling programs.
After heating, a tiltable transfer buggy brings a hot ingot to a two-high reversing mill, which takes one pass after another, reversing the rolls and roller table each time the ingot has passed through. Because each pass reduces the slab by only about 50 millimetres, it may take 21 passes, including several edge passes with the slab standing upright on its edges, to obtain a slab measuring 0.2 metre thick, 1.5 metres wide, and 10 metres long.
The rolls usually have a diameter of about 1.2 metres; each is driven by one or two electric motors totaling 7,000 to 12,000 horsepower. The two roller tables, situated in front and in back of the stand, have movable manipulators that guide the slab into the rolls and turn it onto its edges when required. High-pressure water nozzles remove surface scale, and a crop-shear discards the ends and cuts the slab into proper length. Some slabbing mills place a pair of heavy vertical rolls next to the horizontal rolls for edge rolling; this avoids the time-consuming turning of the slab into an upright position. Such an arrangement is called a universal mill.
For making long products, blooms some 250 millimetres square are rolled from ingots in a similar fashion on the same type of mill.
Plates
Rolled from heavy slabs supplied by a slabbing mill or continuous caster or sometimes rolled directly from an ingot, plates vary greatly in dimensions. The largest mills can roll plates 200 millimetres thick, 5 metres wide, and 35 metres long. These three dimensions are determined by the slab or ingot weight as well as the rolling-mill size. Sometimes only a few plates of the same dimensions and quality specifications are ordered.
 Most mills have two continuous, broadside push-through or walk-through furnaces, which heat the slabs to about 1,250° C. Sometimes two batch-type furnaces are also used for heating odd-sized or extra-heavy slabs and ingots. Before rolling, high-pressure water jets descale the slabs. Most plate mills are four-high mills, as shown in C in the figure-->
Most mills have two continuous, broadside push-through or walk-through furnaces, which heat the slabs to about 1,250° C. Sometimes two batch-type furnaces are also used for heating odd-sized or extra-heavy slabs and ingots. Before rolling, high-pressure water jets descale the slabs. Most plate mills are four-high mills, as shown in C in the figure--> , and are supplemented by vertical edge rolls. The work rolls and backup rolls of large mills have diameters of 1.2 and 2.4 metres, respectively, and a roll face length up to 6 metres. Their maximum total rolling force is often 10,000 tons, and their rolls are driven by an 8,000-kilowatt motor. Most mills have hydraulic roll adjustment, which transmits the roll pressure to a computer; the computer uses this and other rolling parameters, such as temperature and thickness of the plate at all locations, to control the rolling process by a mathematical model. This technology—actually a computerized art—permits not only the rolling of huge workpieces with high accuracy (e.g., to a thickness tolerance of 0.2 millimetre) but also the control of rectangularity, flatness, plan-view shape, yield, physical properties, and profile. Several plants are even capable of rolling plates with a tapered or stepped thickness. Sometimes plants use two rolling mills, a roughing stand and a finishing stand, to improve surface quality and increase production. Most plate mills also have elaborate equipment for leveling, cooling, shearing or milling of edges, heat-treating, and marking.
, and are supplemented by vertical edge rolls. The work rolls and backup rolls of large mills have diameters of 1.2 and 2.4 metres, respectively, and a roll face length up to 6 metres. Their maximum total rolling force is often 10,000 tons, and their rolls are driven by an 8,000-kilowatt motor. Most mills have hydraulic roll adjustment, which transmits the roll pressure to a computer; the computer uses this and other rolling parameters, such as temperature and thickness of the plate at all locations, to control the rolling process by a mathematical model. This technology—actually a computerized art—permits not only the rolling of huge workpieces with high accuracy (e.g., to a thickness tolerance of 0.2 millimetre) but also the control of rectangularity, flatness, plan-view shape, yield, physical properties, and profile. Several plants are even capable of rolling plates with a tapered or stepped thickness. Sometimes plants use two rolling mills, a roughing stand and a finishing stand, to improve surface quality and increase production. Most plate mills also have elaborate equipment for leveling, cooling, shearing or milling of edges, heat-treating, and marking.Hot strip
The rolling of hot strip begins with a slab, which is inspected and, if necessary, surface cleaned either manually or by scarfing machines with oxyacetylene torches. The slabs are then pushed, or walked on their broadside, through gas-fired furnaces that have a hearth dimension of about 13 metres by 30 metres. In a pusher-type furnace, the slabs slide on water-cooled skids, and, each time a new slab is charged, a heated slab drops through a discharge door onto a roller table. In walking-beam furnaces, several walking beams lift the workpieces from the hearth, move them forward, and set them back down in a series of rectangular movements. These furnaces have the advantage of producing no cold stripes and skid marks across the slabs. Preheating temperature, as with slabs and plates, is about 1,250° C.
A heated slab moves first through a scale breaker, which is a two-high rolling mill with vertical rolls that loosens the furnace scale and removes it with high-pressure water jets. Then the slab passes through four-high roughing stands, typically four arranged in tandem, which roll it to a thickness of about 30 millimetres. The stands are spaced about 30 to 70 metres apart, so that the slab is only in one roll gap at a time. After roughing, it proceeds to a long (about 140 metres) roller table in front of the finishing train for cooling, when required for metallurgical reasons. As the slab enters the finishing train (at about 20 metres per minute), a crop-shear cuts the head and tail, and high-pressure steam jets remove the secondary scale formed during rolling. Six or seven four-high finishing stands then roll the strip to its final thickness of 1.5 to 10 millimetres.
Finishing stands are arranged in tandem, only five to six metres apart and close-coupled, so that the strip is in all rolls at the same time. For process control, a computer receives continuous information from on-line sensors, measuring such parameters as thickness, temperature, tension, width, speed, and shape of the strip, as well as roll pressure, torque, and electrical load. Reduction is high in the first stands (e.g., 45 percent) and low in the last stand (e.g., 10 percent) to ensure good surface and flatness of the strip, which leaves the last finishing stand at 600 to 1,200 metres per minute and 820° to 950° C (1,510° to 1,750° F). The strip is water-cooled on a 150-metre-long run-out table and coiled at high speed at 520° to 720° C (970° to 1,325° F). Mills have at least two coilers to ensure 100 percent availability.
All the equipment in a hot-strip mill is arranged in a straight line of about 600 metres from furnace to coiler, with the slab or strip passing only once through each stand. Total installed power of only the heavy rolling-mill motors can exceed 125,000 horsepower.
Controlling rolling and coiling temperatures is essential for metallurgical reasons, because it greatly influences the physical properties of both hot-rolled and cold-rolled strip. Also, a number of systems are in use to improve dimensional control of the strip. In order to guide the strip through the flat rolls of a tandem mill, it is made thicker in the centre (by about 0.1 millimetre) than at the edges. This so-called crown, as well as the strip's entire profile, is often controlled by roll bending, accomplished by hydraulic cylinders and extra-long bearings on each side of the extended roll neck. Another system, which improves the wear pattern and service time of the work rolls, is roll shifting—i.e., a sideward adjustment of the rolls along their axes. Normally, the rolling program of a hot-strip mill is influenced by roll wear. Since the heaviest roll wear takes place at the colder edges of the strip, it is common to roll wide strips first and narrow strips later. Roll shifting permits so-called schedule-free rolling—i.e., strip of any width can be rolled at any time. It also is used for controlling the strip profile.
 Many highly mechanized hot-strip mills have a capacity of three million to five million tons per year, and as much as 60 percent of the raw steel produced in industrial countries is rolled on these mills. There are, however, hot-strip mills designed for smaller production. For example, a semi-continuous hot-strip mill has only one reversing rougher in front of the finishing train. Another rolling system goes even farther and uses one four-high reversing rougher and one four-high reversing finishing mill, with hot-coiling boxes in front and in back of the finishing mill. (Hot coilers operate in a furnace to keep the strip hot.) In addition, there are planetary-type hot-strip mills, which have a cage of approximately 20 small rolls around each of two backup rolls (see F in the figure-->
Many highly mechanized hot-strip mills have a capacity of three million to five million tons per year, and as much as 60 percent of the raw steel produced in industrial countries is rolled on these mills. There are, however, hot-strip mills designed for smaller production. For example, a semi-continuous hot-strip mill has only one reversing rougher in front of the finishing train. Another rolling system goes even farther and uses one four-high reversing rougher and one four-high reversing finishing mill, with hot-coiling boxes in front and in back of the finishing mill. (Hot coilers operate in a furnace to keep the strip hot.) In addition, there are planetary-type hot-strip mills, which have a cage of approximately 20 small rolls around each of two backup rolls (see F in the figure--> ). The small rolls, in turning around the big roll, make a small reduction every time they pass over the wedge-shaped portion of the workpiece in the roll gap. Planetary mills can reduce a slab from 25 to 2.5 millimetres in one pass—although at a slow rate.
). The small rolls, in turning around the big roll, make a small reduction every time they pass over the wedge-shaped portion of the workpiece in the roll gap. Planetary mills can reduce a slab from 25 to 2.5 millimetres in one pass—although at a slow rate.Cold strip
The rolling of cold strip begins with the retrieval of hot-rolled strip from a coil storage yard, which often uses fully automated cranes for setting and retrieving coils according to rolling schedules. The coils are first descaled in continuous pickle lines, which are discussed below (see Treating of steel: Surface treating: Pickling (steel)). The cleaned and oiled coils are fed into a cold-reduction mill, which is usually a tandem mill of four to six four-high stands with an uncoiling reel at the entry and a recoiling reel at the exit. When rolling from, for example, 2 millimetres to 0.3 millimetre, the cold reduction is usually 35 percent on the first stands and 15 percent on the final stand. The exit speed is normally high, often 100 kilometres (60 miles) per hour, in order to achieve proper production rates with such small cross sections. Since the strip temperature may go as high as 200° C (390° F), proper cooling of strip and rolls is essential. Heavy-duty lubricants are also used to minimize friction in the roll gap.
 Typically, the work rolls have a diameter of a half-metre, and the backup rolls of 1.2 metres. For wide strip, the roll face can be 2.4 metres long. The work rolls are precision ground with a specific crown to compensate for roll bending. The last stand usually takes only a small reduction to improve control over the final thickness, profile, and flatness of the strip. To improve control further, many shops use hydraulic roll bending, or they use a differential cooling of the rolls to change their shape by thermal expansion. For additional shape control, a number of shops employ a six-high mill (D in the figure-->
Typically, the work rolls have a diameter of a half-metre, and the backup rolls of 1.2 metres. For wide strip, the roll face can be 2.4 metres long. The work rolls are precision ground with a specific crown to compensate for roll bending. The last stand usually takes only a small reduction to improve control over the final thickness, profile, and flatness of the strip. To improve control further, many shops use hydraulic roll bending, or they use a differential cooling of the rolls to change their shape by thermal expansion. For additional shape control, a number of shops employ a six-high mill (D in the figure--> ) as the last stand, shifting the work rolls and intermediate rolls along their axes during rolling. This provides continuous shape control, because the rolls are ground to a specific profile. All these systems, together with the high speed of rolling, make cold-reduction mills highly complex to operate and controllable only by computer.
) as the last stand, shifting the work rolls and intermediate rolls along their axes during rolling. This provides continuous shape control, because the rolls are ground to a specific profile. All these systems, together with the high speed of rolling, make cold-reduction mills highly complex to operate and controllable only by computer.Usually, cold-rolled strip cannot be used as rolled, because it is too hard and has low ductility. Therefore, it is annealed in batch or continuous annealing plants (see below Treating of steel: Heat-tresting: Annealing (steel)). After annealing, the strip is cold-rolled to about a 3-percent reduction on a temper mill to improve its physical properties. (Temper mills are dry, four-high reversing mills that are similar to cold-reduction mills but less powerful.) This rolling operation also gives the strips their final surface finish, an important characteristic and often specified by the customer. If required, shearing lines cut the coils into sheets.
Several plants integrate some or all of the operating steps of a cold-rolling shop into a continuous operation, moving an endless strip (welded together at the pickler or cold mill) through the processes without coiling and coil storage. Indeed, some plants move one continuous strip from the pickle line to the temper-mill exit, with cold-rolling and annealing in between. One of these continuous lines can take less than two hours to convert a hot-rolled coil into a shippable cold-rolled product—a great operating advantage that requires, however, excellent computer control at all levels and perfect maintenance to provide the needed reliability for the completely linked-up equipment. With direct charging of a hot-strip mill from a continuous caster, it is possible to have liquid steel in shippable form five hours after it has been tapped at the furnace.
Billets, bars, and rods
Billets

 Billets are the feedstock for long products of small cross section. In cases when they are not directly cast by a continuous caster, they are rolled from blooms by billet mills. One method of rolling billets, which are usually 75 to 125 millimetres square, is to use a three-high mill with box passes, as shown in A in the figure-->
Billets are the feedstock for long products of small cross section. In cases when they are not directly cast by a continuous caster, they are rolled from blooms by billet mills. One method of rolling billets, which are usually 75 to 125 millimetres square, is to use a three-high mill with box passes, as shown in A in the figure--> . After a rectangular bloom is rolled into a square cross section at the lower rolls, it is lifted to the next pass on the upper rolls and rolled back into a rectangular one; this is turned 90° while being lowered on a roller table for another square rolling in the lower pass, and so on. In another method, alternating horizontal and vertical stands are arranged in tandem, using diamond and square passes without turning or twisting the billet (as shown in B in the figure-->
. After a rectangular bloom is rolled into a square cross section at the lower rolls, it is lifted to the next pass on the upper rolls and rolled back into a rectangular one; this is turned 90° while being lowered on a roller table for another square rolling in the lower pass, and so on. In another method, alternating horizontal and vertical stands are arranged in tandem, using diamond and square passes without turning or twisting the billet (as shown in B in the figure--> ).
).Bars

 Bars are long products, usually of round, square, rectangular, or hexagonal cross section and of 12- to 50-millimetre diameter or equivalent. (Since bar mills are also capable of rolling small shaped products such as angles, flats, channels, fence posts, and tees, these products are sometimes called merchant bars.) In rolling bars, a billet measuring, for instance, 120 millimetres square and five metres long is heated in pusher or walking-beam furnaces to 1,200° C. Τhere is a great variety of layouts used in bar-rolling mills. In principle, after removal of the furnace scale by water jets, a primary reduction takes place in several passes through roll stands in open, semicontinuous, or fully continuous arrangement. These can use an alternating square-diamond rolling principle on horizontal and vertical rolls, as shown in B in the figure-->
Bars are long products, usually of round, square, rectangular, or hexagonal cross section and of 12- to 50-millimetre diameter or equivalent. (Since bar mills are also capable of rolling small shaped products such as angles, flats, channels, fence posts, and tees, these products are sometimes called merchant bars.) In rolling bars, a billet measuring, for instance, 120 millimetres square and five metres long is heated in pusher or walking-beam furnaces to 1,200° C. Τhere is a great variety of layouts used in bar-rolling mills. In principle, after removal of the furnace scale by water jets, a primary reduction takes place in several passes through roll stands in open, semicontinuous, or fully continuous arrangement. These can use an alternating square-diamond rolling principle on horizontal and vertical rolls, as shown in B in the figure--> , or a series of oval-to-round passes, as illustrated in C in the figure-->
, or a series of oval-to-round passes, as illustrated in C in the figure--> .
. Guiding the strands properly from roll gap to roll gap is an important part of this rolling technology. When using only horizontal rolls, the guides also twist the bar 90° between diamond and square passes. In a continuous arrangement of close-coupled mills—in which several roll pairs or roll sets are installed a short distance from one another and all are driven through gears by one or two motors—bars are allowed to buckle in a controlled vertical loop in order to maintain a low tension in a bar between the stands. When using an open-train arrangement, a U-shaped trough called the repeater guides and threads the strand, as indicated in G in the figure-->
Guiding the strands properly from roll gap to roll gap is an important part of this rolling technology. When using only horizontal rolls, the guides also twist the bar 90° between diamond and square passes. In a continuous arrangement of close-coupled mills—in which several roll pairs or roll sets are installed a short distance from one another and all are driven through gears by one or two motors—bars are allowed to buckle in a controlled vertical loop in order to maintain a low tension in a bar between the stands. When using an open-train arrangement, a U-shaped trough called the repeater guides and threads the strand, as indicated in G in the figure--> . This generates a horizontal loop, caused by the entry speed of each receiving stand being slower than the exit speed of the delivering stands.
. This generates a horizontal loop, caused by the entry speed of each receiving stand being slower than the exit speed of the delivering stands.The finishing stand of a bar mill gives the bar its final shape and often a specific surface pattern, such as the protrusions on concrete-reinforcing bars. The rolling speed increases as the cross section at each successive stand decreases, and the exit speed can be as high as 15 metres per second. The hot bar is then cut by a flying shear into cooling-bed length (e.g., 50 metres), after which it is cooled, inspected, and cold-cut to shipping length.
Rods
Rod mills are similar to bar mills at the front end, but the finishing end is different. Rods have a smaller section (5.5 to 15 millimetres in diameter) and are always coiled, while bars are normally shipped in cut length. The final rolling in rod mills often takes place in a close-coupled set of 10 pairs of small rolls (200 and 150 millimetres in diameter); these are all installed in a block, with their axes at a 45° angle and arranged in an alternating fashion like the vertical and horizontal rolls in a continuous bar mill. Exit speed of small-diameter rods can go up to 100 metres per second. The rod is immediately coiled by quickly rotating laying heads and cooled before bundling. For enhanced production, two strands are often rolled simultaneously. Such high-speed operation requires cooling of the rod and almost every rolling-mill component. The cooling condition of the bars and rods is also carefully controlled to meet metallurgical specifications.
Computers are used for designing roll passes and for scheduling and controlling the complex operations. Bar and rod mills produce 150,000 to 750,000 tons per year. The largest mills are housed in buildings up to 600 metres long. The most space-consuming part of these manufacturing facilities (and the source of most bottlenecks) is the finishing and shipping area, which handles the many different lightweight shapes that are produced in various steel grades, heat treatments, and surface conditions and are made to many specific customer orders.
Shapes
These are long products with irregular cross sections, such as beams, channels, angles, and rails. Rolling starts with blooms that may be 150 millimetres by 200 millimetres by 5 metres long. The blooms are received, either cold or hot, directly from the blooming mill or continuous caster. They are charged into a pusher or walking-beam continuous furnace and heated for up to three hours to 1,200° C. (Sometimes, three batch-type furnaces are used instead.)
 Most shapes are formed by grooved rolls with mating projections that form together a window in their gap. This window becomes progressively smaller and more like the desired shape, pass after pass, until at the end, in the final pass, the specified cross section is obtained. D in the figure-->
Most shapes are formed by grooved rolls with mating projections that form together a window in their gap. This window becomes progressively smaller and more like the desired shape, pass after pass, until at the end, in the final pass, the specified cross section is obtained. D in the figure--> shows only 5 progressive passes out of about 11 in the rolling of a rail. Rolling shapes usually takes a total of 9 to 15 passes, with an area reduction of about 25 percent at the initial passes and only 7 percent at the last pass.
shows only 5 progressive passes out of about 11 in the rolling of a rail. Rolling shapes usually takes a total of 9 to 15 passes, with an area reduction of about 25 percent at the initial passes and only 7 percent at the last pass. Roll and pass design is critical for this rolling technology. There are usually three to five stands arranged in various ways, each taking one to five passes. Only one pass is made through the finishing stand, which controls the final dimension and surface. Sometimes two-high reversing mills are used at the beginning in a fashion similar to blooming mills, with manipulators on run-out roller tables. In other cases, two or three three-high, nonreversing stands are arranged as an open train; in this arrangement, lifting roller tables move the workpiece between the upper and lower pass lines, and the workpiece is in only one roll gap at a time. Mills that produce medium and small shapes often have stands in tandem arrangement, rolling one workpiece simultaneously in several stands and using a controlled loop between stands. Wide-flange I-beams and H-pilings are usually rolled on universal mills using vertical edgers, as indicated in E in the figure-->
Roll and pass design is critical for this rolling technology. There are usually three to five stands arranged in various ways, each taking one to five passes. Only one pass is made through the finishing stand, which controls the final dimension and surface. Sometimes two-high reversing mills are used at the beginning in a fashion similar to blooming mills, with manipulators on run-out roller tables. In other cases, two or three three-high, nonreversing stands are arranged as an open train; in this arrangement, lifting roller tables move the workpiece between the upper and lower pass lines, and the workpiece is in only one roll gap at a time. Mills that produce medium and small shapes often have stands in tandem arrangement, rolling one workpiece simultaneously in several stands and using a controlled loop between stands. Wide-flange I-beams and H-pilings are usually rolled on universal mills using vertical edgers, as indicated in E in the figure--> . Blooms with a dog-bone cross section are often supplied to these structural-shape mills by beam-blank continuous casters.
. Blooms with a dog-bone cross section are often supplied to these structural-shape mills by beam-blank continuous casters.Rolling temperatures are carefully controlled for metallurgical reasons. Heavy-walled, wide-flange I-beams are sometimes heat-treated in-line by computer-controlled water quenching and by tempering with their own retained heat. The heads of rails are often heat-treated in-line to improve wear and impact resistance. Rails are also slow-cooled under an insulated cover, directly after rolling, for at least 10 hours to diffuse hydrogen out of the steel.
After rolling, a hot saw cuts the shapes into lengths that can be handled by the cooling bed. Each shop conducts large-size finishing operations such as straightening, cold-cutting to ordered length, marking, and inspection.
Tubes
Tubular products are manufactured according to two basic technologies. One is the welding of tubes from strip, and the other is the production of seamless tube from rounds or blooms.
Welded tubes
 The most widely used welding system, the electric-resistance welding (ERW) line, starts with a descaled hot-rolled strip that is first slit into coils of a specific width to fit a desired tube diameter. In the entry section is an uncoiler, a welder that joins the ends of coils for continuous operation, and a looping pit, which permits constant welding rates of, typically, three metres per minute. Several consecutive forming rolls then shape the strip into a tube with a longitudinal seam on top, as shown schematically in A in the figure-->
The most widely used welding system, the electric-resistance welding (ERW) line, starts with a descaled hot-rolled strip that is first slit into coils of a specific width to fit a desired tube diameter. In the entry section is an uncoiler, a welder that joins the ends of coils for continuous operation, and a looping pit, which permits constant welding rates of, typically, three metres per minute. Several consecutive forming rolls then shape the strip into a tube with a longitudinal seam on top, as shown schematically in A in the figure--> . Two squeeze rolls press the seam together, while two electrode rolls or sliding contacts feed the electric power to the seam for resistance heating and welding. A cutting tool removes the flash created during welding, and, after a preliminary inspection, the tube is cut into cooling-bed length by a saw that moves with the tube.
. Two squeeze rolls press the seam together, while two electrode rolls or sliding contacts feed the electric power to the seam for resistance heating and welding. A cutting tool removes the flash created during welding, and, after a preliminary inspection, the tube is cut into cooling-bed length by a saw that moves with the tube. Tubes up to 500 millimetres in diameter with walls 10 millimetres thick are produced on ERW lines. Larger-diameter pipes are often produced by forming the strip into an endless spiral, as shown schematically in B in the figure-->
Tubes up to 500 millimetres in diameter with walls 10 millimetres thick are produced on ERW lines. Larger-diameter pipes are often produced by forming the strip into an endless spiral, as shown schematically in B in the figure--> . Forming is followed by continuous welding of the seam, often by automatic arc welding. Pipes up to 1.5 metres in diameter and with a 12-millimetre wall thickness are sometimes produced by this spiral welding process. Still larger pipes are produced from plates by a U-ing and O-ing process, which applies heavy presses to form plates into a U and then an O. The longitudinal seam (or seams) are then welded by automatic arc-welding equipment.
. Forming is followed by continuous welding of the seam, often by automatic arc welding. Pipes up to 1.5 metres in diameter and with a 12-millimetre wall thickness are sometimes produced by this spiral welding process. Still larger pipes are produced from plates by a U-ing and O-ing process, which applies heavy presses to form plates into a U and then an O. The longitudinal seam (or seams) are then welded by automatic arc-welding equipment.Seamless tubes
 Seamless tube rolling always begins by piercing a round or bloom to generate a hollow. In roll piercing, an oval round is preheated to about 1,200° C and is cross-rolled slowly between two short, large-diameter rolls that rotate in the same direction (shown schematically in C in the figure-->
Seamless tube rolling always begins by piercing a round or bloom to generate a hollow. In roll piercing, an oval round is preheated to about 1,200° C and is cross-rolled slowly between two short, large-diameter rolls that rotate in the same direction (shown schematically in C in the figure--> ). The round also revolves and is pulled into the roll gap in a spiraling motion, because the rolls have a converging-diverging shape and are installed relative to each other at an angle of about 20°. This revolving, continuous plastic working of an oval cross section between the two rolls creates tensile stresses in the long axes of the oval, which rupture the centre and create a cavity. At this point the cavity meets the piercer, which is a projectile-shaped rotating cone held in place by a bar and a thrust bearing. The piercer acts like a third roll in the centre and produces the inside of the tube.
). The round also revolves and is pulled into the roll gap in a spiraling motion, because the rolls have a converging-diverging shape and are installed relative to each other at an angle of about 20°. This revolving, continuous plastic working of an oval cross section between the two rolls creates tensile stresses in the long axes of the oval, which rupture the centre and create a cavity. At this point the cavity meets the piercer, which is a projectile-shaped rotating cone held in place by a bar and a thrust bearing. The piercer acts like a third roll in the centre and produces the inside of the tube. The cross or helical rolling action of roll piercing demands excellent hot formability of the prerolled round. Another process, push piercing, does not have such exacting requirements. This usually takes continuously cast square blooms and forms them into hollow rounds by the action of a heavy hydraulic pusher, which pushes them into the gap of two large-diameter contoured rolls that form together a circular pass line. In the roll gap the bloom is met by a heavy piercer, which forms the hollow, as shown in D in the figure-->
The cross or helical rolling action of roll piercing demands excellent hot formability of the prerolled round. Another process, push piercing, does not have such exacting requirements. This usually takes continuously cast square blooms and forms them into hollow rounds by the action of a heavy hydraulic pusher, which pushes them into the gap of two large-diameter contoured rolls that form together a circular pass line. In the roll gap the bloom is met by a heavy piercer, which forms the hollow, as shown in D in the figure--> . This mill can form a 250-millimetre-square, 3-metre-long bloom into a tube with an outside diameter of 300 millimetres and an inside diameter of 150 millimetres. Since there are only compression forces acting on the steel in this process, the workpiece is practically not elongated at all.
. This mill can form a 250-millimetre-square, 3-metre-long bloom into a tube with an outside diameter of 300 millimetres and an inside diameter of 150 millimetres. Since there are only compression forces acting on the steel in this process, the workpiece is practically not elongated at all. A number of rolling technologies are used to form the pierced hollows into tubes with specific dimensions and tolerances. Often, the hollow is reheated and then sent through another cross-roll piercer mill, called the elongator; this reduces the wall thickness by 30 to 60 percent. In a subsequent step, a long, preheated, lubricated cylinder called a mandrel may be inserted into the tube. The tube would then be rolled, with the mandrel inside, in a continuous close-coupled, seven-stand, two-high mill, usually with the rolls arranged at a 45° angle and in an alternating pattern like the horizontal and vertical rolls. A very uniform wall thickness can be formed by this process. Smaller diameter tubes are often formed from larger tubes in a continuous three-roll, close-coupled stretch-reduction mill (E in the figure-->
A number of rolling technologies are used to form the pierced hollows into tubes with specific dimensions and tolerances. Often, the hollow is reheated and then sent through another cross-roll piercer mill, called the elongator; this reduces the wall thickness by 30 to 60 percent. In a subsequent step, a long, preheated, lubricated cylinder called a mandrel may be inserted into the tube. The tube would then be rolled, with the mandrel inside, in a continuous close-coupled, seven-stand, two-high mill, usually with the rolls arranged at a 45° angle and in an alternating pattern like the horizontal and vertical rolls. A very uniform wall thickness can be formed by this process. Smaller diameter tubes are often formed from larger tubes in a continuous three-roll, close-coupled stretch-reduction mill (E in the figure--> ). These mills sometimes have 20 sets of rolls arranged in tandem.
). These mills sometimes have 20 sets of rolls arranged in tandem.Open-die forging
Heavy ingots, some weighing up to 300 tons, are sometimes formed at steel plants by huge hydraulic presses with a forging force of up to 10,000 tons. These make such large products as rotors for power-generating units or large sleeves for rolls or pressure vessels. Careful, uniform heating of the ingots to forging temperature may take 60 hours, and, before completion of the forging process, the workpiece may be reheated six times. The forging is accomplished by flat-, vee-, or swage-shaped dies, depending on the shape of the final product. Saddles and mandrels are used for forging rings and sleeves. The workpiece is connected to a long bar, which helps to move and turn it by a crane or manipulator. Large heat-treating furnaces are available in these forging shops to improve microstructure and to release internal stresses caused by the forging operation.
Wire
The cold drawing of wire is an important and special sector of steelmaking. It produces wire in hundreds of sizes and shapes and within a spectrum of physical properties unmatched by other steel products. Wire is also produced with many types of surface finish.
Treating of steel
Heat-treating
 In principle, heat-treating already takes place when steel is hot-rolled at a particular temperature and cooled afterward at a certain rate, but there are also many heat-treating process facilities specifically designed to produce particular microstructures and properties. The simplest heat-treating process is normalizing. This consists of holding steel for a short time at a temperature 20° to 40° C above the G-S-K line (shown in the iron-carbon diagram in the figure-->
In principle, heat-treating already takes place when steel is hot-rolled at a particular temperature and cooled afterward at a certain rate, but there are also many heat-treating process facilities specifically designed to produce particular microstructures and properties. The simplest heat-treating process is normalizing. This consists of holding steel for a short time at a temperature 20° to 40° C above the G-S-K line (shown in the iron-carbon diagram in the figure--> ) and then cooling it afterward in still air. Holding the steel in the gamma zone transforms the as-rolled or as-cast microstructure into austenite, which dissolves carbides. Then, during cooling, a very uniform grain is formed, consisting of either pearlite and ferrite or pearlite and cementite, depending on carbon content.
) and then cooling it afterward in still air. Holding the steel in the gamma zone transforms the as-rolled or as-cast microstructure into austenite, which dissolves carbides. Then, during cooling, a very uniform grain is formed, consisting of either pearlite and ferrite or pearlite and cementite, depending on carbon content.In all heat-treatment operations, the temperatures, holding times, and heating and cooling rates are varied according to the chemical composition, size, and shape of the steel. In general, alloy steels, which have a lower heat conductivity than carbon steels, are heated more slowly to avoid internal stresses.
annealing
 To make steel ductile for subsequent forming operations, an annealing treatment is applied. In annealing, the steel is usually held for several hours at several degrees below Ar1 (shown by the P-S-K line in the figure-->
To make steel ductile for subsequent forming operations, an annealing treatment is applied. In annealing, the steel is usually held for several hours at several degrees below Ar1 (shown by the P-S-K line in the figure--> ) and then slowly cooled. This precipitates and coagulates the carbides and results in large ferrite crystals. Cold-formed steel is usually annealed and recrystallized in this manner, holding it for several hours at about 680° C (1,260° F).
) and then slowly cooled. This precipitates and coagulates the carbides and results in large ferrite crystals. Cold-formed steel is usually annealed and recrystallized in this manner, holding it for several hours at about 680° C (1,260° F).Annealing is performed in an inert or reducing atmosphere to prevent any oxidation of the steel surface. In batch annealing of cold-rolled strip, for example, several coils are set on a base and on top of one another. Then they are covered with a shell made of heat-resistant steel, which is sealed on the bottom and holds the inert gas during annealing. A gas-fired bell furnace is then lowered by a crane over this cover for heating. The total processing time, including cooling, may be 50 to 120 hours, depending on furnace load and steel grade.
In a different system, the cold-rolled strip is pulled through an 80-metre-high furnace with the strip moving up and down between many top and bottom rolls. These continuous-annealing furnaces are usually heated by gas-fired radiation tubes in order to separate combustion gases from the inert atmosphere surrounding the strip. In this dynamic annealing process, the strip is heated to higher temperatures (for example, 780° C, or 1,440° F), held for only a few seconds, and immediately cooled by fast-circulating inert gas. The entry and exit sections of continuous-annealing lines are built, as on other strip-processing lines, to allow an uninterrupted and constant travel (at, say, 500 metres per minute) of the strip through the process section—in this case, the heating and cooling zones. The entry group has two uncoiling reels, a cross-shear, welding equipment for joining two strips, and a strip accumulator. The latter is often a looping tower, which supplies the process section above with strip at constant speed while welding is done at the entry section. The exit group works in a similar fashion, with a looping tower and two reels; it also cuts samples and substandard portions out of the strip.
Continuous-annealing lines are often 200 metres long, and the strip between uncoiler and recoiler is more than one kilometre in length. Strip annealed this way is not as soft as batch-annealed steel—a disadvantage compensated for by using ultralow-carbon steels—but it does have operating advantages in that annealing of one coil may take only one hour and the mechanical and surface properties of the strip are very uniform.
quenching and tempering
The most common heat treatment for plates, tubular products, and rails is the quench-and-temper process. Large plates are heated in roller-type or walking-beam furnaces, quenched in special chambers, and then tempered in a separate low-temperature furnace. Uniform heating and quenching is crucial; otherwise, residual stresses will distort and warp the plate. Tubes made for very demanding services, such as oil drilling, are usually heat-treated in walking-beam furnaces and special quench-and-temper systems.
The heads of rails are sometimes heat-treated in-line by induction heating coils, air quenching, and tempering by a controlled use of the heat retained in the rail after quenching. Heavy-walled structural shapes are sometimes water-quenched directly after the last pass at the rolling mill and also tempered by the heat retained in the steel. In-line heat-treating results in cost savings because it eliminates extra heat-treating processes and facilities.
The quenching media and the type of agitation during quenching are carefully selected to obtain specified physical properties with minimum internal stresses and distortions. Oil is the mildest medium, and salt brine has the strongest quenching effect; water is between the two. In special cases, steel is cooled and held for some time in a molten salt bath, which is kept at a temperature either just above or just below the temperature where martensite begins to form. These two heat treatments are called martempering and austempering, and both result in even less distortion of the metal.
Surface treating
The surface treatment of steel also begins during hot-rolling, because reheating conditions, in-line scale removal, rolling temperature, and cooling rate all determine the type and thickness of scale formed on the product, and this affects atmospheric corrosion, paintability, and subsequent scale-removal operations. Sometimes the final pass in hot-rolling generates specific surface patterns—for example, the protrusions on reinforcing bars or floor plates—and in cold-rolling a specific surface roughness is rolled into the strip at the temper mill to improve the deep-drawing operation and to assure a good surface finish on the final product—for instance, on the roof of an automobile.
Pickling
Before cold forming, hot-rolled steel is always descaled, most commonly in an operation known as pickling. Scale consists of thin layers of iron oxide crystals, of which the chemical compositions, structures, and densities vary according to the temperature, oxidizing conditions, and steel properties that are present during their formation. These crystals can be dissolved by acids; normally, hot hydrochloric or sulfuric acid is used, but for some alloy steels a different acid, such as nitric acid, is needed. In addition, inhibitors are added to the acid to protect the steel from being dissolved as well.
The pickling of hot-rolled strip is carried out in continuous pickle lines, which are sometimes 300 metres long. The strip is pulled through three to five consecutive pickling tanks, each one 25 to 30 metres long, at a constant speed of about 300 metres per minute. Like other continuous strip-processing lines, pickle lines also have an entry and exit group to establish constant pickling conditions. After the last acid tank, there are sections that rinse, neutralize, dry, inspect, and oil the strip.
Long products, such as bars and wire rods, are normally pickled in batch operations by placing them on racks and immersing them in long, acid-containing vats. Sometimes shotblasting is used instead of pickling; this removes scale from heavy hot-rolled products by directing high-velocity abrasives onto the surface of the steel.
Cleaning
The removal of organic substances and other residues from the surface of steel, in particular after cold forming with lubricants, is carried out either in special cleaning lines or in the cleaning sections of another processing line. Hot solutions of caustic soda, phosphates, or alkaline silicates are used. The strip is often moved through several sets of electrodes, which, submerged in the cleaning liquid, electrolytically generate hydrogen gas at the steel surface for lifting residues off the strip.
Surface coating
Approximately one-third of the steel shipped by the industry is coated on its surface by a metallic, inorganic, or organic coating. By far the largest installations are operated for coating cold-rolled strip. In this group the most widely used are those which coat the steel with zinc, zinc alloys, or aluminum.
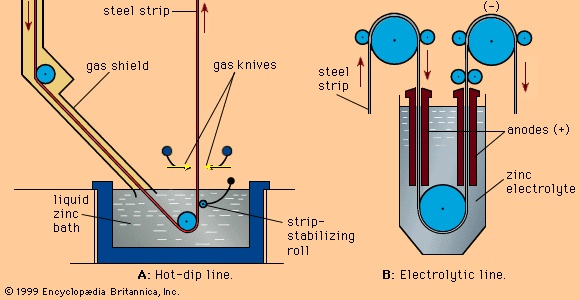 In hot-dip galvanizing lines, which also have the usual entry and exit groups, the strip moves first at constant speed—say, 150 metres per minute—through a cleaning section and a long, horizontal, nonoxidizing preheating furnace. (When hard strips are coated directly after cold reduction, this furnace is also used for annealing.) The hot strip, still protected by the inert furnace atmosphere in a long steel channel, enters the zinc bath at a temperature of approximately 480° C (900° F), supplying heat to the zinc bath, which is at about 440° C (825° F). The liquid zinc is contained in a refractory-lined, induction-heated vessel called the zinc pot (shown schematically in A in the figure-->
In hot-dip galvanizing lines, which also have the usual entry and exit groups, the strip moves first at constant speed—say, 150 metres per minute—through a cleaning section and a long, horizontal, nonoxidizing preheating furnace. (When hard strips are coated directly after cold reduction, this furnace is also used for annealing.) The hot strip, still protected by the inert furnace atmosphere in a long steel channel, enters the zinc bath at a temperature of approximately 480° C (900° F), supplying heat to the zinc bath, which is at about 440° C (825° F). The liquid zinc is contained in a refractory-lined, induction-heated vessel called the zinc pot (shown schematically in A in the figure--> ). When it contacts the strip surface, the liquid zinc alloys with the iron and forms a strong metallurgical bond. However, the iron-zinc alloy is brittle, so that the coating, if too thick, will crack during forming of the sheet. For this reason, about 0.1 to 0.25 percent aluminum is added to the zinc, inhibiting iron-zinc formation and keeping the alloy layer to less than 15 percent of the total coating thickness. Excess liquid zinc is wiped off each side of the strip by two gas-knives, which have long, slotlike orifices through which high-pressure gas is blown. Coating thickness is controlled by adjusting the gas pressure and the location of the knives. Common coating weights are 180 or 275 grams of zinc per square metre of sheet, counting both surfaces. Sometimes, a heavy coating is produced on one side and a lighter coating on the other; this is called a differential coating. The total length of hot-dip galvanizing lines, including furnaces and cooling zones, sometimes reaches 400 metres. The entire system is computer-controlled, based on the continuous, in-line measuring of the coating weight.
). When it contacts the strip surface, the liquid zinc alloys with the iron and forms a strong metallurgical bond. However, the iron-zinc alloy is brittle, so that the coating, if too thick, will crack during forming of the sheet. For this reason, about 0.1 to 0.25 percent aluminum is added to the zinc, inhibiting iron-zinc formation and keeping the alloy layer to less than 15 percent of the total coating thickness. Excess liquid zinc is wiped off each side of the strip by two gas-knives, which have long, slotlike orifices through which high-pressure gas is blown. Coating thickness is controlled by adjusting the gas pressure and the location of the knives. Common coating weights are 180 or 275 grams of zinc per square metre of sheet, counting both surfaces. Sometimes, a heavy coating is produced on one side and a lighter coating on the other; this is called a differential coating. The total length of hot-dip galvanizing lines, including furnaces and cooling zones, sometimes reaches 400 metres. The entire system is computer-controlled, based on the continuous, in-line measuring of the coating weight.There are several variations of the basic galvanizing process. The galvanneal process heats the strip above the zinc pot right after coating, using induction coils or gas-fired burners to create a controlled, heavy iron-zinc layer for improved weldability, abrasion resistance, and paintability of the product. Several processes use a zinc-aluminum alloy, and some lines have a second pot filled with liquid aluminum for aluminum coating. The pots are often quickly exchangeable.
 Electrolytic galvanizing lines have similar entry and exit sections, but they deposit zinc in as many as 20 consecutive electrolytic coating cells. Of the several successful cell designs, the simple vertical cell (B in the figure-->
Electrolytic galvanizing lines have similar entry and exit sections, but they deposit zinc in as many as 20 consecutive electrolytic coating cells. Of the several successful cell designs, the simple vertical cell (B in the figure--> ) is discussed here to explain the principle. The strip, connected to the negative side of a direct current through large-diameter conductor rolls located above and between two cells, is dipped into a tank of electrolyte by a submerged sink roll. Partially submerged anodes, opposing the strip, are connected to the positive side of the electric current by heavy bus bars. Zinc cations (i.e., positively charged zinc atoms) present in the electrolyte are converted by the current into regular zinc atoms, which deposit on the strip. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously added to the electrolyte. In the latter case the anodes are made of insoluble materials, such as titanium coated with iridium oxide. The electrolyte is an acidic solution of zinc sulfide or zinc chloride with other bath additions to improve the quality of the coating and the current efficiency. Coating thickness is easier to control here than in the hot-dip process because of the good relationship between electrical current and deposited zinc. Theoretically, 1.22 kilograms of zinc are formed when applying a current of 1,000 amperes over one hour; this means that a line with an installed electrical capacity of one million amperes can deposit 1.22 tons of zinc per hour. The control parameters of such a line are mainly the current density between anodes and strip, the line voltage, the chemical composition and temperature of the electrolyte, and the line speed.
) is discussed here to explain the principle. The strip, connected to the negative side of a direct current through large-diameter conductor rolls located above and between two cells, is dipped into a tank of electrolyte by a submerged sink roll. Partially submerged anodes, opposing the strip, are connected to the positive side of the electric current by heavy bus bars. Zinc cations (i.e., positively charged zinc atoms) present in the electrolyte are converted by the current into regular zinc atoms, which deposit on the strip. The bath is supplied with zinc cations either by zinc anodes, which are continuously dissolved by the direct current, or by zinc compounds continuously added to the electrolyte. In the latter case the anodes are made of insoluble materials, such as titanium coated with iridium oxide. The electrolyte is an acidic solution of zinc sulfide or zinc chloride with other bath additions to improve the quality of the coating and the current efficiency. Coating thickness is easier to control here than in the hot-dip process because of the good relationship between electrical current and deposited zinc. Theoretically, 1.22 kilograms of zinc are formed when applying a current of 1,000 amperes over one hour; this means that a line with an installed electrical capacity of one million amperes can deposit 1.22 tons of zinc per hour. The control parameters of such a line are mainly the current density between anodes and strip, the line voltage, the chemical composition and temperature of the electrolyte, and the line speed.Electrolytic lines normally produce lower coating weights (15 to 60 grams per square metre) than do hot-dip lines, and they can also easily supply differential coatings and one-sided coatings for specific applications. Many lines can deposit zinc-alloy coatings, such as zinc-nickel or zinc-iron, and some lines are capable of producing multilayered coatings of different alloys, the goal being to optimize a combination of specific requirements such as corrosion resistance, weldability, abrasion resistance, drawability, and paintability. The processing speed of electrolytic galvanizing lines can often reach 180 metres per minute.
Electrolytic tinning lines for the production of tinplate are, in principle, of similar design, except that all rolls are smaller (because the strip is thinner and narrower), the line speed is faster (e.g., 700 metres per minute), and different electrolytes and anodes are used. Electrolytic coating lines also coat strips with chromium and other metals and alloys. Most of these lines have a shear line installed at the end to produce cut-to-length sheets upon request.
Many long products are also surface coated. Wires, for example, are often hot-dip galvanized in continuous multistrand lines. In addition, electrolytic coating of wire with all types of metal is often done by hanging coils from current-carrying C-hooks or bars into long vats, which have anodes installed and are filled with electrolyte. Many tubular products and reinforcing bars are coated with organic material to inhibit corrosion.
History
The steel industry has grown from ancient times, when a few men may have operated, periodically, a small furnace producing 10 kilograms, to the modern integrated iron- and steelworks, with annual steel production of about 1 million tons. The largest commercial steelmaking enterprise, Nippon Steel (Nippon Steel Corporation) in Japan, was responsible for producing 26 million tons in 1987, and 11 other companies generally distributed throughout the world each had outputs of more than 10 million tons. Excluding the Eastern-bloc countries, for which employment data are not available, some 1.7 million people were employed in 1987 in producing 430 million tons of steel. That is equivalent to about 250 tons of steel per person employed per year—a remarkably efficient use of human endeavour.
Primary steelmaking
Early iron and steel
Iron production began in Anatolia about 2000 BC, and the Iron Age was well established by 1000 BC. The technology of iron making then spread widely; by 500 BC it had reached the western limits of Europe, and by 400 BC it had reached China. Iron ores are widely distributed, and the other raw material, charcoal, was readily available. The iron was produced in small shaft furnaces as solid lumps, called blooms, and these were then hot forged into bars of wrought iron, a malleable material containing bits of slag and charcoal.
The carbon contents of the early irons ranged from very low (0.07 percent) to high (0.8 percent), the latter constituting a genuine steel. When the carbon content of steel is above 0.3 percent, the material will become very hard and brittle if it is quenched in water from a temperature of about 850° to 900° C (1,550° to 1,650° F). The brittleness can be decreased by reheating the steel within the range of 350° to 500° C (660° to 930° F), in a process known as tempering. This type of heat treatment was known to the Egyptians by (Egypt, ancient) 900 BC, as can be judged by the microstructure of remaining artifacts, and formed the basis of a steel industry for producing a material that was ideally suited to the fabrication of swords and knives.
The Chinese (China) made a rapid transition from the production of low-carbon iron to high-carbon cast iron, and there is evidence that they could produce heat-treated steel during the early Han dynasty (206 BC–AD 25). The Japanese (Japan) acquired the art of metalworking from the Chinese, but there is little evidence of a specifically Japanese steel industry until a much later date.
The Romans (ancient Rome), who have never been looked upon as innovators but more as organizers, helped to spread the knowledge of iron making, so that the output of wrought iron in the Roman world greatly increased. With the decline of Roman influence, iron making continued much as before in Europe, and there is little evidence of any change for many centuries in the rest of the world. However, by the beginning of the 15th century, waterpower was used to blow air into bloomery furnaces; as a consequence, the temperature in the furnace increased to above 1,200° C (2,200° F), so that, instead of forming a solid bloom of iron, a liquid was produced rich in carbon—i.e., cast iron. In order to make this into wrought iron by reducing the carbon content, solidified cast iron was passed through a finery, where it was melted in an oxidizing atmosphere with charcoal as the fuel. This removed the carbon to give a semisolid bloom, which, after cooling, was hammered into shape.
Blister steel
In order to convert wrought iron into steel—that is, increase the carbon content—a carburization process was used. Iron billets were heated with charcoal in sealed clay pots that were placed in large bottle-shaped kilns holding about 10 to 14 tons of metal and about 2 tons of charcoal. When the kiln was heated, carbon from the charcoal diffused into the iron. In an attempt to achieve homogeneity, the initial product was removed from the kiln, forged, and again reheated with charcoal in the kiln. During the reheating process, carbon monoxide gas was formed internally at the nonmetallic inclusions; as a result, blisters formed on the steel surface—hence the term blister steel to describe the product. This process spread widely throughout Europe, where the best blister steel was made with Swedish wrought iron. One common steel product was weapons. To make a good sword, the carburizing, hammering, and carburizing processes had to be repeated about 20 times before the steel was finally quenched and tempered and made ready for service. Thus, the material was not cheap.
About the beginning of the 18th century, coke produced from coal began to replace charcoal as the fuel for the blast furnace; as a result, cast iron became cheaper and even more widely used as an engineering material. The Industrial Revolution then led to an increased demand for wrought iron, which was the only material available in sufficient quantity that could be used for carrying loads in tension. One major problem was the fact that wrought iron was produced in small batches. This was solved about the end of the 18th century by the puddling process, which converted the readily available blast-furnace iron into wrought iron. In Britain by 1860 there were 3,400 puddling furnaces producing a total of 1.6 million tons per year—about half the world's production of wrought iron. Only about 60,000 tons were converted into blister steel in Britain; annual world production of blister steel at this time was about 95,000 tons. Blister steel continued to be made on a small scale into the 20th century, the last heat taking place at Newcastle, Eng., in 1951.
Crucible steel
A major development occurred in 1751, when Benjamin Huntsman (Huntsman, Benjamin) established a steelworks at Sheffield, Eng., where the steel was made by melting blister steel in clay crucibles at a temperature of 1,500° to 1,600° C (2,700° to 2,900° F), using coke as a fuel. Originally, the charge in the crucible weighed about 6 kilograms, but by 1870 it had increased to 30 kilograms, which, with a crucible weight of 10 kilograms, was the maximum a man could be expected to lift from a hot furnace. The liquid metal was cast to give an ingot about 75 millimetres in square section and 500 millimetres long, but multiple casts were also made. Sheffield became the centre of crucible steel production; in 1873, the peak year, output was 110,000 tons—about half the world's production. The crucible process spread to Sweden and France following the end of the Napoleonic Wars and then to Germany, where it was associated with Alfred Krupp's works in Essen. A small crucible steelworks was started in Tokyo in 1895, and crucible steel was produced in Pittsburgh, Pa., U.S., from 1860, using a charge of wrought iron and pig iron.
The crucible process allowed alloy steels to be produced for the first time, since alloying elements could be added to the molten metal in the crucible, but it went into decline from the early 20th century, as electric-arc furnaces became more widely used. It is believed that the last crucible furnace in Sheffield was operated until 1968.
Bessemer (Bessemer, Sir Henry) steel
Bulk steel production was made possible by Henry Bessemer in 1855, when he obtained British patents for a pneumatic steelmaking process. (A similar process is said to have been used in the United States by William Kelly in 1851, but it was not patented until 1857.) Bessemer used a pear-shaped vessel lined with ganister, a refractory material containing silica, into which air was blown from the bottom through a charge of molten pig iron. Bessemer realized that the subsequent oxidation of the silicon and carbon in the iron would release heat and that, if a large enough vessel were used, the heat generated would more than offset the heat lost. A temperature of 1,650° C (3,000° F) could thus be obtained in a blowing time of 15 minutes with a charge weight of about half a ton.
One difficulty with Bessemer's process (Bessemer process) was that it could convert only a pig iron low in phosphorus and sulfur. (These elements could have been removed by adding a basic flux such as lime, but the basic slag produced would have degraded the acidic refractory lining of Bessemer's converter.) While there were good supplies of low-phosphorus iron ores (mostly hematite) in Britain and the United States, they were more expensive than phosphorus-rich ores. In 1878 Sidney Gilchrist Thomas and Percy Gilchrist developed a basic-lined converter in which calcined dolomite was the refractory material. This enabled a lime-rich slag to be used that would hold phosphorus and sulfur in solution. This “basic Bessemer” process was little used in Britain and the United States, but it enabled the phosphoric ores of Alsace and Lorraine to be used, and this provided the basis for the development of the Belgian, French, and German steel industries. World production of steel rose to about 50 million tons by 1900.
The open hearth
An alternative steelmaking process was developed in the 1860s by William and Friedrich Siemens in Britain and Pierre and Émile Martin in France. The open-hearth furnace was fired with air and fuel gas that were preheated by combustion gases to 800° C (1,450° F). A flame temperature of about 2,000° C (3,600° F) could be obtained, and this was sufficient to melt the charge. Refining—that is, removal of carbon, manganese, and silicon from the metal—was achieved by a reaction between the slag (to which iron ore was added) and the liquid metal in the hearth of the furnace. Initially, charges of 10 tons were made, but furnace capacity gradually increased to 100 tons and eventually to 300 tons. Initially an acid-lined furnace was used, but later a basic process was developed that enabled phosphorus and sulfur to be removed from the charge. A heat could be produced in 12 to 18 hours, sufficient time to analyze the material and adjust its composition before it was tapped from the furnace.
The great advantage of the open hearth was its flexibility: the charge could be all molten pig iron, all cold scrap, or any combination of the two. Thus, steel could be made away from a source of liquid iron. Up to 1950, 90 percent of steel in Britain and the United States was produced in the open-hearth process, and as recently as 1988 more than 96 million tons per year were produced in this way by Eastern-bloc countries.
Oxygen steelmaking
The refining of steel in the conventional open-hearth furnace required time-consuming reactions between slag and metal. After World War II, tonnage oxygen became available, and many attempts were made to speed up the steelmaking process by blowing oxygen directly into the charge. The Linz-Donawitz (LD) process, developed in Austria in 1949, blew oxygen through a lance into the top of a pear-shaped vessel similar to a Bessemer converter. Since there was no cooling effect from inert nitrogen gas present in air, any heat not lost to the off-gas could be used to melt scrap added to the pig-iron charge. In addition, by adding lime to the charge, it was possible to produce a basic slag that would remove phosphorus and sulfur. With this process, which became known as the basic oxygen process (BOP), it was possible to produce 200 tons of steel from a charge consisting of up to 35 percent scrap in a tap-to-tap time of 60 minutes. The charges of a basic oxygen furnace have grown to 400 tons and, with a low-silicon charge, blowing times can be reduced to 15 to 20 minutes.
Shortly after the introduction of the LD process, a modification was developed that involved blowing burnt lime through the lance along with the oxygen. Known as the LD-AC (after the ARBED steel company of Luxembourg and the Centre National of Belgium) or the OLP (oxygen-lime powder) process, this led to the more effective refining of pig iron smelted from high-phosphorus European ores. A return to the original bottom-blown Bessemer concept was developed in Canada and Germany in the mid-1960s; this process used two concentric tuyeres with a hydrocarbon gas in the outer annulus and oxygen in the centre. Known originally by the German abbreviation OBM (for Oxygen bodenblasen Maxhuette, “oxygen bottom-blowing Maxhuette”), it became known in North America as the Q-BOP. Beginning about 1960, all oxygen steelmaking processes replaced the open-hearth and Bessemer processes on both sides of the Atlantic.
Electric steelmaking
With the increasing sophistication of the electric power industry toward the end of the 19th century, it became possible to contemplate the use of electricity as an energy source in steelmaking. By 1900, small electric-arc furnaces capable of melting about one ton of steel were introduced. These were used primarily to make tool steels, thereby replacing crucible steelmaking. By 1920 furnace size had increased to a capacity of 30 tons. The electricity supply was three-phase 7.5 megavolt-amperes, with three graphite electrodes being fed through the roof and the arcs forming between the electrodes and the charge in the hearth. By 1950 furnace capacity had increased to 50 tons and electric power to 20 megavolt-amperes.
Although small arc furnaces were lined with acidic refractories, these were little more than melting units, since hardly any refining occurred. The larger furnaces were basic-lined, and a lime-rich slag was formed under which silicon, sulfur, and phosphorus could be removed from the melt. The furnace could be operated with a charge that was entirely scrap or a mixture of scrap and pig iron, and steel of excellent quality with sulfur and phosphorus contents as low as 0.01 percent could be produced. The basic electric-arc process was therefore ideally suited for producing low-alloy steels and by 1950 had almost completely replaced the basic open-hearth process in this capacity. At that time, electric-arc furnaces produced about 10 percent of all the steel manufactured (about 200 million tons worldwide), but, with the subsequent use of oxygen to speed up the basic arc process, basic electric-arc furnaces accounted for almost 30 percent of steel production by 1989. In that year, world steel production was 770 million tons.
Secondary steelmaking
With the need for improved properties in steels, an important development after World War II was the continuation of refining in the ladle after the steel had been tapped from the furnace. The initial developments, made during the period 1950–60, were to stir the liquid in the ladle by blowing a stream of argon through it. This had the effect of reducing variations in the temperature and composition of the metal, allowing solid oxide inclusions to rise to the surface and become incorporated in the slag, and removing dissolved gases such as hydrogen, oxygen, and nitrogen. Gas stirring alone, however, could not remove hydrogen to an acceptable level when casting large ingots. With the commercial availability after 1950 of large vacuum pumps, it became possible to place ladles in large evacuated chambers and then, by blowing argon as before, remove hydrogen to less than two parts per million. Between 1955 and 1965 a variety of improved degassing systems of this type were developed in Germany.
The oldest ladle addition treatment was the Perrin process developed in 1933 for removing sulfur. The steel was poured into a ladle already containing a liquid reducing slag, so that violent mixing occurred and sulfur was transferred from the metal to the slag. The process was expensive and not very efficient. In the postwar period, desulfurizing powders based on calcium, silicon, and magnesium were injected into the liquid steel in the ladle through a lance using an inert carrier gas. This method was pioneered in Japan to produce steels for gas and oil pipelines.
Alloying (alloy)
Alloying elements are added to steels in order to improve specific properties such as strength, wear, and corrosion resistance. Although theories of alloying have been developed, most commercial alloy steels have been developed by an experimental approach with occasional inspired guesses. The first experimental study of alloy additions to steel was made in 1820 by the Britons James Stodart and Michael Faraday, who added gold and silver to steel in an attempt to improve its corrosion resistance. The mixtures were not commercially feasible, but they initiated the idea of adding chromium to steel (see below Stainless steel).
Hardening and strengthening
The first commercial alloy steel is usually attributed to the Briton Robert F. Mushet, who in 1868 discovered that adding tungsten to steel greatly increased its hardness even after air cooling. This material formed the basis of the subsequent development of tool steels for the machining of metals.
About 1865 Mushet also discovered that the addition of manganese to Bessemer steel enabled the casting of ingots free of blowholes. He was also aware that manganese alleviated the brittleness induced by the presence of sulfur, but it was Robert Hadfield who developed (in 1882) a steel containing 12 to 14 percent manganese and 1 percent carbon that greatly improved wear resistance and was used for jaw crushers and railway crossover points.
The real driving force for alloy steel development was armaments. About 1889 a steel was produced with 0.3 percent carbon and 4 percent nickel; shortly thereafter it was further improved by the addition of chromium and became widely used for armour plate on battleships. In 1918 it was found that this steel could be made less brittle by the addition of molybdenum.
The general understanding of why or how alloying elements influenced the depth of hardening—the hardenability—came out of research conducted chiefly in the United States during the 1930s. An understanding of why properties changed on tempering came about in the period 1955–1965, following the use of the transmission electron microscope.
Microalloyed steels
An important development immediately after World War II was the improvement of steel compositions for plates and sections that could readily be welded. The driving force for this work was the failure of plates on the Liberty ships mass-produced during the war by welding, a faster fabricating process than riveting. The improvements were effected by increasing the manganese content to 1.5 percent and keeping the carbon content below 0.25 percent.
A group of steels given the generic title high-strength low-alloy (HSLA) steels had the similar aim of improving the general properties of mild steels with small additions of alloying elements that would not greatly increase the cost. By 1962 the term microalloyed steel was introduced for mild-steel compositions to which 0.01 to 0.05 percent niobium had been added. Similar steels were also produced containing vanadium.
The period 1960–80 was one of considerable development of microalloyed steels. By linking alloying with control over temperature during rolling, yield strengths were raised to almost twice that of conventional mild steel.
Stainless steels (stainless steel)
It is not surprising that attempts should be made to improve the corrosion resistance of steel by the addition of alloying elements, but it is surprising that a commercially successful material was not produced until 1914. This was a composition of 0.4 percent carbon and 13 percent chromium, developed by Harry Brearley in Sheffield for producing cutlery.
Chromium was first identified as a chemical element about 1798 and was extracted as an iron-chromium-carbon alloy. This was the material used initially by Stodart and Faraday in 1820 in their experiments on alloying. The same material was used by John Woods and John Clark in 1872 to make an alloy containing 30 to 35 percent chromium; although it was noted as having improved corrosion resistance, the steel was never exploited. Success became possible when Hans Goldschmidt, working in Germany, discovered in 1895 how to make low-carbon ferrochromium.
The link between the carbon content of chromium steels and their corrosion resistance was established in Germany by Philip Monnartz in 1911. During the interwar period, it became clearly established that there had to be at least 8 percent chromium dissolved in the iron matrix (and not bound up with carbon in the form of carbides), so that on exposure to air a protective film of chromic oxide would form on the steel surface. In Brearley's steel, 3.5 percent of the chromium was tied up with the carbon, but there was still sufficient remaining chromium to confer corrosion resistance.
The addition of nickel to stainless steel was patented in Germany in 1912, but the materials were not exploited until 1925, when a steel containing 18 percent chromium, 8 percent nickel, and 0.2 percent carbon came into use. This material was exploited by the chemical industry from 1929 onward and became known as the 18/8 austenitic grade.
By the late 1930s there was a growing awareness that the austenitic stainless steels were useful for service at elevated temperatures, and modified compositions were used for the early jet aircraft engines produced during World War II. The basic compositions from that period are still in use for high-temperature service. Duplex stainless steel was developed during the 1950s to meet the needs of the chemical industry for high strength linked to corrosion resistance and wear resistance. These alloys have a microstructure consisting of about half ferrite and half austenite and a composition of 25 percent chromium, 5 percent nickel, 3 percent copper, and 2 percent molybdenum.
Forming and casting
The early metals shapers, the smiths, used hand tools to form iron into finished shapes. Essentially, these consisted of tongs for holding the metal on an anvil and a hammer for beating it. Converting an iron bloom into a wrought-iron bar required considerable hammering. Water-driven hammers were in use by the 15th century in Germany, but heavy hammers capable of dealing with 100-kilogram blooms came into use only in the 18th century. Slitting mills for making thin strips that were then fabricated into nails were introduced about that time, as were rolling mills for converting bars into flat plates. Grooved rolls for producing rods from puddled iron were patented by John Purnell in 1766; these were powered by a 35-horsepower waterwheel.
casting
Steel-forming operations were on a relatively small scale until the introduction of the Bessemer process, in which large volumes of liquid steel were produced for the first time. The liquid metal was poured from ladles into large cast-iron ingot molds with an average size of 700 millimetres in square section and 1.5 to 2 metres in length. Such an ingot would weigh about seven tons. After solidifying, the ingot was stripped from the mold, reheated, and then reduced in size by hot-rolling in a primary (blooming) mill to give billets about 100 millimetres in section. The billets were sheared into 3- to 4-metre lengths, and these formed the starting material for rolling into bars, beams, rods, and strip.
This type of billet production persisted until the 1960s, when a profound change occurred with the development of continuous-casting machines. With liquid steel going directly from the furnace into the casting machine, there was no need to pour large ingots or to reheat them with heavy energy requirements. Nor were the very expensive blooming mills required for reducing the ingots to forms that were now produced directly by casting. Continuous casting was first used for nonferrous metals in the 1930s, and in the early 1950s experiments were undertaken with it at steel plants in Britain, the United States, and the U.S.S.R. The first production plant using continuous casting was operated at Barrow, Eng., by the United Steels Company. In 1965, 2 percent of total steel production was continuously cast; by 1970 this had risen to 5 percent, and, by 1990, 64 percent of all the steel produced in the world was continuously cast (in Japan it was more than 90 percent).
Continuous casting was partly responsible for a new type of steel plant that developed after 1970—the so-called mini-mill. There steel was made in an electric-arc furnace using an all-scrap charge and was then continuously cast into small-diameter billets for rolling into rods or drawing into wire. Mini-mills were built in industrial regions, where scrap arises, whereas the location of conventional steel plants remained linked to the availability of iron ore and low-cost energy.
Tubes
With the development of the gas industry at the beginning of the 19th century, an increased demand developed for tubes to transmit gas. In 1824 a method for pressure butt-welding of heated, curved strip was developed in Britain, and in 1832 a plant for producing tubes was established in the United States. Similar processes are still being used to produce seamed tubing. An improvement on the hot-pressure butt-weld was developed in the United States about 1921, when the seam was joined by electric-resistance welding. Most seamed tubes are still produced this way, including the large-diameter tubes formed by spirally coiling a continuous strip and then arc welding the spiral seam.
Seamless tubing involved the piercing of a round billet; this process was developed in Britain in 1841. A greatly improved process was developed by the Mannesmann company in Germany in 1886; this involved rolling the billet longitudinally and at the same time forcing it onto a piercing bar called a mandrel. The method is widely used for both ferrous and nonferrous metals.
forging
As the size of ingots increased in the late 19th century, large hammer forges were developed that simulated the early blacksmiths' hammering action. For really large components, the first press forge was built in Britain in 1861 and introduced into the United States by 1877. In these forges, the upper forging die is pressed against the workpiece on the lower anvil by a hydraulically operated piston.
Foundry (founding)
The introduction of the crucible process enabled steel castings to be produced for the first time. Steel products were being cast in Germany and Switzerland from 1824, and, by 1855, steel gear wheels were cast in Sheffield. In the United States, steel castings were first produced in Pittsburgh in 1871.
The crucible process continued to be the chief melting method until 1893, when the Tropenas converter, a side-blown, Bessemer-type vessel, was developed in Sheffield. Electric melting in acid-lined furnaces was pioneered in Switzerland in 1907, and electric furnace melting is now predominantly used for making steel castings.
Research on molding sands (which have a great influence on the quality of steel castings) started in the United States in 1919, and this led to the publishing of international standards for molding materials during the period 1924–28. X-ray methods for assessing the soundness of steel castings were introduced in the U.S.S.R. in the 1920s, and magnetic crack-detection methods followed in the 1930s.
Plates and sheet
Plates are produced by hot-rolling, the technology for which developed in the early 19th century. In order to produce sheet from plate, the steel is cold-rolled, and, as there is a limit to the reduction in thickness that can be achieved by one pass through a rolling stand, a series of stands are arranged in tandem. The first mill of this type was installed in 1904 in the United States.
In making wide, thin sheets, difficulties arise because the small-diameter rolls necessary for producing thin material have a tendency to bend in service, giving a sheet that is thicker in the middle than at the edges. The problems were overcome after World War II by the introduction of larger-diameter backup rolls. In an extreme case, the cluster mill, each small work roll was backed by nine larger-diameter supporting rolls.
World steel production
Steel (raw) Steel (raw)The table provides a list of raw steel production by country.
Additional Reading
Comprehensive and up-to-date information on many aspects of metallurgy, individual metals, and alloys can be found in convenient reference-form arrangement in the following works: Metals Handbook, 9th ed., 17 vol. (1978–89), a massive and detailed source prepared under the direction of the American Society for Metals, with a 10th edition that began publication in 1990; Herman F. Mark et al. (eds.), Encyclopedia of Chemical Technology, 3rd ed., 31 vol. (1978–84), formerly known as Kirk-Othmer Encyclopedia of Chemical Technology, with a 4th edition begun in 1991; and its European counterpart, the first English-language edition of a monumental German work, Ullmann's Encyclopedia of Industrial Chemistry, 5th, completely rev. ed., edited by Wolfgang Gerhartz et al. (1985– ). Ed. Information on the properties of a wide range of steels is given in R.W.K. Honeycombe, Steels: Microstructures and Properties (1981). William T. Lankford, Jr., et al. (eds.), The Making, Shaping, and Treating of Steel, 10th ed. (1985), provides a comprehensive survey of all steelmaking technologies; for descriptions of the important liquid steelmaking processes in a more compact form, see C. Moore and R.I. Marshall, Modern Steelmaking Methods (1980). The theory, design, and operation of basic oxygen methods are discussed in R.D. Pehlke et al. (eds.), BOF Steelmaking, 5 vol. (1974–77). Clarence E. Sims (ed.), Electric Furnace Steelmaking, 2 vol. (1962–63), discusses all aspects of this method. Charles R. Taylor (ed.), Electric Furnace Steelmaking (1985); and International Iron And Steel Institute Committee On Technology, The Electric Arc Furnace, 1990 (1990), cover more recent developments in the field. Secondary steelmaking methods are discussed in R.J. Fruehan, Ladle Metallurgy Principles and Practices (1985). For information on all aspects of an important solidification method, see Continuous Casting (1983– ), published by the Iron & Steel Society of the American Institute of Mining, Metallurgical, and Petroleum Engineers (AIME). All important rolling methods are covered in Vladimir B. Ginzburg, Steel-Rolling Technology: Theory and Practice (1989). The best English-language books covering all aspects of surface treating and heat treating are relevant volumes of Metals Handbook, cited above.Major products of the industry are covered in the Steel Products Manual (irregular), published by the American Iron and Steel Institite of the AIME. Conference proceedings and periodical publications of societies and institutions all over the world concerned specifically with steelmaking, steel products, and use of steel provide detailed state-of-the-art information—for example, Steel Technology International (annual); Steel Today and Tomorrow (five times a year); and Steel Times International (bimonthly). K.C. Barraclough, Steelmaking Before Bessemer, 2 vol. (1984), is a history of steelmaking prior to 1850, focusing on blister steel and crucible steel, and Steelmaking: 1850–1900 (1990), is an account of the development of the Bessemer and open-hearth processes. Henry Bessemer, Sir Henry Bessemer, F.R.S.: An Autobiography (1905, reprinted 1989), provides relevant information in Bessemer's own account of his life and work. There is no specific historical work on post-1950 developments in oxygen steelmaking, but a concise account of these newer processes is offered in C. Moore and R.I. Marshall, Steelmaking (1991). Relevant information is found in the materials of Historical Metallurgy (semiannual), the journal of the British Historical Metallurgy Society.
- Joseph of Volokolamsk, Saint
- Joseph Olbrich
- Joseph Othmar von Rauscher
- Joseph Papp
- Joseph Paul-Boncour
- Joseph Paxson Iddings
- Joseph P. Bradley
- Joseph Pennell
- Joseph Pickett
- Joseph Pitton de Tournefort
- Joseph P. Kennedy
- Joseph Priestley
- Joseph Profaci
- Joseph Proud
- Joseph Pulitzer
- Joseph Radetzky, Graf
- Joseph Redlich
- Joseph Rheinberger
- Joseph R. McCarthy
- Joseph Roberts Smallwood
- Joseph Rodman Drake
- Joseph Rogers Brown
- Joseph Roth
- Joseph Roumanille
- Joseph Rucker Lamar