curium
chemical element
(Cm),
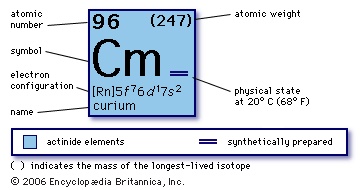 synthetic chemical element of the actinoid series of the periodic table, atomic number 96. Undetected in nature, curium (as the isotope curium-242) was discovered (summer 1944) at the University of Chicago by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in a plutonium isotope, plutonium-239, that had been bombarded by helium ions (alpha particles) in the 60-inch cyclotron at the University of California, Berkeley. It was the third transuranium element to be discovered.
synthetic chemical element of the actinoid series of the periodic table, atomic number 96. Undetected in nature, curium (as the isotope curium-242) was discovered (summer 1944) at the University of Chicago by Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in a plutonium isotope, plutonium-239, that had been bombarded by helium ions (alpha particles) in the 60-inch cyclotron at the University of California, Berkeley. It was the third transuranium element to be discovered.Curium is a silvery metal. All of its isotopes are radioactive. For chemical research curium-242 (163-day half-life) has been supplanted by curium-244 (17.6-year half-life) and the still longer-lived isotopes from curium-245 to curium-248, all of which are built up from plutonium-239 by neutron irradiation. Curium exhibits its common +3 oxidation state as the very faint yellow Cm3+ ion in aqueous solution, as the sesquioxide Cm2O3, and as the trihalides; it is chemically similar to the other tripositive actinide elements and to the lanthanide elements. The +4 oxidation state appears in the black dioxide CmO2 and as the Cm4+ ion complexed with the fluoride ion. The isotopes curium-242 and curium-244 are well suited for use in space because they can provide compact, long-lived sources of electricity through conversion of their heat of radioactive decay by thermoelectric and thermionic devices.
atomic number
96
stablest isotope
247
melting point
c. 1,340° C (2,444° F)
specific gravity
c. 13.51
oxidation states
+3, +4
electronic config.
【Rn】5f 76d17s2
- bola
- Bolama
- Boland, Eavan
- Bolcom, William
- Bolden, Buddy
- Boldogasszony
- Boldrewood, Rolf
- bolero
- Boleslav I
- Boleslav II
- Boleslav III
- Bolesław Bierut
- Bolesław I
- Bolesław II
- Bolesław III
- Bolesław Leśmian
- Bolesław Prus
- Bolesławski, Richard
- Boletaceae
- Boletales
- Bolgary
- Bolgatanga
- Bolger, James Brendan
- Bolingbroke, Henry Saint John, 1st Viscount, Baron Saint John Of Lydiard Tregoze
- Bolivia