platinum
chemical element
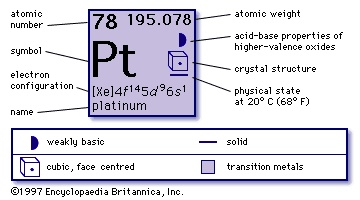 chemical element, the best known and most widely used of the six platinum metals (platinum group) of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table. A very heavy, precious, silver-white metal, platinum is soft and ductile and has a high melting point and good resistance to corrosion and chemical attack. For example, its surface remains bright after being brought to white heat in air, and, though it readily dissolves in aqua regia, it is scarcely attacked by simple acids. (It does dissolve slowly in hydrochloric acid in the presence of air.) Small amounts of iridium are commonly added to give a harder, stronger alloy that retains the advantages of pure platinum.
chemical element, the best known and most widely used of the six platinum metals (platinum group) of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table. A very heavy, precious, silver-white metal, platinum is soft and ductile and has a high melting point and good resistance to corrosion and chemical attack. For example, its surface remains bright after being brought to white heat in air, and, though it readily dissolves in aqua regia, it is scarcely attacked by simple acids. (It does dissolve slowly in hydrochloric acid in the presence of air.) Small amounts of iridium are commonly added to give a harder, stronger alloy that retains the advantages of pure platinum.Platinum, one of the most abundant platinum metals, and its alloys are indispensable in the chemical laboratory for electrodes and for crucibles and dishes in which materials can be heated to high temperatures. Platinum is used for electrical contacts and sparking points because it resists both the high temperatures and chemical attack of electric arcs. Jewelry and dental alloys account for much of its use; platinum-iridium is used for surgical pins. The prototype international standard kilogram of mass was made from an alloy of 90 percent platinum and 10 percent iridium. The electrical resistivity of platinum is relatively high and depends markedly upon the temperature; the international temperature scale from −183° to 630° C (−297° to 1,166° F) is defined in terms of a resistance thermometer made with platinum wire. As a catalyst, platinum has many applications, notably in automotive catalytic converters and in petroleum refining.
The Italian-French physician Julius Caesar Scaliger (Scaliger, Julius Caesar) alluded (1557) to a refractory metal, probably platinum, found between Darién and Mexico. The first certain discovery was in the alluvial deposits of the Río Pinto, Colombia. The Spaniards called the new metal platina del Pinto for its resemblance to silver. The world's most important deposits occur in the Transvaal of South Africa. Other deposits are found in Russia, Finland, Ireland, Borneo, New South Wales, New Zealand, Brazil, Peru, and Madagascar. In North America native platinum is found in Alaska, California, and Oregon, in British Columbia, and in Alberta. Placer deposits are the most productive sources of the native element. The ordinary variety of native platinum is called polyxene; it is 80 percent to 90 percent platinum, with 3 percent to 11 percent iron, plus the other platinum metals, and gold, copper, and nickel. For mineralogical properties, see native element (table). Platinum is also found in the very rare native alloy platiniridium. Platinum occurs combined with arsenic as sperrylite (PtAs2) in the copper–nickel-mining district near Sudbury, Ont., and with sulfur as cooperite (PtS) in the Transvaal. (For information about the mining, recovery, and production of platinum, see platinum processing (platinum group).)
Platinum is rapidly attacked by fused alkali oxides and peroxides and also by fluorine and chlorine at about 500° C. It is capable of absorbing large volumes of hydrogen, and, with palladium, it is one of the most reactive platinum metals.
Platinum forms an important series of compounds with the oxidation states of +2 and +4. Many of these compounds contain coordination complexes in which chloride ion (Cl−), ammonia (NH3), or other groups are bonded to a central platinum atom. Among the transition metals, platinum has one of the greatest tendencies to form bonds directly with carbon. Platinum also combines with a number of nonmetallic elements on heating, such as phosphorus, arsenic, antimony, silicon, sulfur, and selenium.
Natural platinum is a mixture of six isotopes: platinum-190 (0.0127 percent), platinum-192 (0.78 percent), platinum-194 (32.9 percent), platinum-195 (33.8 percent), platinum-196 (25.3 percent), and platinum-198 (7.21 percent). All are stable except platinum-190 and platinum-192, which have been reported as long-lived alpha emitters.
atomic number
78
atomic weight
195.09
melting point
1,769° C (3,216° F)
boiling point
3,827° C (6,920° F)
specific gravity
21.45 (20° C)
oxidation states
+2, +4
electronic config.
【Xe】4f145d96s1
- Thomas Brown
- Thomas B Thorpe
- Thomas Butler, 10th earl of Ormonde
- Thomas Butler Ormonde, 10th earl of
- Thomas Campbell
- Thomas Campion
- Thomas, Cardinal Wolsey
- Thomas Carew
- Thomas Carlyle
- Thomas Cavendish
- Thomas Chalmers
- Thomas Chandler Haliburton
- Thomas Charles
- Thomas Chatterton
- Thomas Chippendale
- Thomas Chippendale, II
- Thomas Chrowder Chamberlin
- Thomas Chubb
- Thomas Churchyard
- Thomas, Clarence
- Thomas Clarkson
- Thomas Clifford, 1st Baron Clifford
- Thomas Clifford Clifford, 1st Baron
- Thomas Cochrane, 10th Earl of Dundonald
- Thomas Cochrane Dundonald, 10th Earl of