poison
nuclear physics
in nuclear physics, any material that can easily capture neutrons without subsequently undergoing nuclear fission. Examples of poisons are the naturally occurring elements boron and cadmium and the fission products xenon-135 and samarium-149. In nuclear reactors, poisons act as parasitic neutron absorbers and lower the rate of fission.
physiology
Introduction
in biochemistry, a substance, natural or synthetic, that causes damage to living tissues and has an injurious or fatal effect on the body, whether it is ingested, inhaled, or absorbed or injected through the skin.
Although poisons have been the subject of practical lore since ancient times, their systematic study is often considered to have begun during the 16th century, when the German-Swiss physician and alchemist Paracelsus first stressed the chemical nature of poisons. It was Paracelsus who introduced the concept of dose and studied the actions of poisons through experimentation. It was not until the 19th century, however, that the Spaniard Matthieu Orfila, the attending physician to Louis XVIII, correlated the chemistry of a toxin with the biological effects it produces in a poisoned individual. Both concepts continue to be fundamental to an understanding of modern toxicology.
Poisoning involves four elements: the poison, the poisoned organism, the injury to the cells, and the symptoms and signs or death. These four elements represent the cause, subject, effect, and consequence of poisoning. To initiate the poisoning, the organism is exposed to the toxic chemical. When a toxic level of the chemical is accumulated in the cells of the target tissue or organ, the resultant injury to the cells disrupts their normal structure or function. Symptoms and toxic signs then develop, and, if the toxicity is severe enough, death may result.
This article considers humans as the primary subjects of poisoning. It first discusses the actions of poisons on the body and then examines principal types of synthetic and natural poisons.
Nature of a toxic substance
Definition of a poison
A poison is a substance capable of producing adverse effects on an individual under appropriate conditions. The term “substance” is almost always synonymous with “chemical” and includes drugs, vitamins, pesticides, pollutants, and proteins. Even radiation is a toxic substance. Though not usually considered to be a “chemical,” most radiations are generated from radioisotopes, which are chemicals. The term “adverse effects” above refers to the injury, such as structural damage to tissues. “Appropriate conditions” refers to the dosage of the substance that is sufficient to cause these adverse effects. The dose concept is important because according to it even a substance as innocuous as water is poisonous if too much is ingested. Whether a drug acts as a therapy or as a poison depends on the dose.
Classification of a poison
Poisons are of such diverse natures that they are classified by origin, physical form, chemical nature, chemical activity, target site, or use.
Classification based on origin
Poisons are of microbial, plant, animal, or synthetic origin. Microbial poisons are produced by microscopic organisms such as bacteria and fungi. Botulinus toxin, for example, is produced by the bacterium Clostridium botulinum and is capable of inducing weakness and paralysis when present in underprocessed, nonacidic canned foods or in other foods containing the spores. An example of a plant toxin is the belladonna alkaloid hyoscyamine, which is found in belladonna (Atropa belladonna) and jimsonweed (Datura stramonium).
Animal poisons are usually transferred through the bites and stings of venomous terrestrial or marine animals, the former group including poisonous snakes, scorpions, spiders, and ants, and the latter group including sea snakes, stingrays, and jellyfish. Synthetic toxins are responsible for most poisonings. “Synthetic” refers to chemicals manufactured by chemists, such as drugs and pesticides, as well as chemicals purified from natural sources, such as metals from ores and solvents from petroleum. Synthetic toxins include pesticides, household cleaners, cosmetics, pharmaceuticals, and hydrocarbons.
Classification based on physical form
The physical form of a chemical—solid, liquid, gas, vapour, or aerosol—influences the exposure and absorbability.
Because solids (solid) are generally not well absorbed into the blood, they must be dissolved in the aqueous liquid lining the intestinal tract if ingested or the respiratory tract if inhaled. Solids dissolve at different rates in fluids, however. For example, compared with lead sulphate granules, granules of lead are practically nontoxic when ingested, because elemental lead is essentially insoluble in water, while lead sulphate is slightly soluble and absorbable. Even different-sized granules of the same chemical can vary in their relative toxicities because of the differences in dissolution rates. For example, arsenic trioxide is more toxic in the form of smaller granules than is the same mass of larger granules because the smaller granules dissolve faster.
A poison in a liquid form can be absorbed by ingestion or by inhalation or through the skin. Poisons that are gases at room temperature (e.g., carbon monoxide) are absorbed mainly by inhalation, as are vapours, which are the gas phase of substances that are liquids at room temperature and atmospheric pressure (e.g., benzene). Because organic liquids are more volatile than inorganic liquids, inhalation of organic vapours is more common. Although vapours are generally absorbed in the lungs, some vapours that are highly soluble in lipids (e.g., furfural) are also absorbed through the skin.
Aerosols (aerosol) are solid or liquid particles small enough to remain suspended in air for a few minutes. Fibres and dust are solid aerosols. Aerosol exposures occur when aerosols are deposited on the skin or inhaled. Aerosol toxicity is usually higher in the lungs than on the skin. An example of a toxic fibre is asbestos, which can cause a rare form of lung cancer (mesothelioma).
Many liquid poisons can exist as liquid aerosols, although highly volatile liquids, such as benzene, seldom exist as aerosols. A moderately volatile liquid poison can exist as both an aerosol and as a vapour. Airborne liquid chemicals of low volatility exist only as aerosols.
Classification based on chemical nature
Poisons can be classified according to whether the chemical is metallic versus nonmetallic, organic versus inorganic, or acidic versus alkaline. Metallic (organometallic compound) poisons are often eliminated from the body slowly and accumulate to a greater extent than nonmetallic poisons and thus are more likely to cause toxicity during chronic exposure. Organic (organic compound) chemicals are more soluble in lipids (lipid) and therefore can usually pass through the lipid-rich cell membranes more readily than can inorganic chemicals. As a result, organic chemicals are generally absorbed more extensively than inorganic chemicals. Classification based on acidity is useful because, while both acids and alkalis are corrosive to the eyes, skin, and intestinal tract, alkalis generally penetrate the tissue more deeply than acids and tend to cause more severe tissue damage.
Classification based on chemical activity
Electrophilic (electron-loving) chemicals attack the nucleophilic (nucleus-loving) sites of the cells' macromolecules, such as deoxyribonucleic acid (DNA), producing mutations, cancers, and malformations. Poisons also may be grouped according to their ability to mimic the structure of certain important molecules in the cell. They substitute for the cells' molecules in chemical reactions, disrupting important cellular functions. Methotrexate, for example, disrupts the synthesis of DNA and ribonucleic acid (RNA).
Other classifications
Unlike the classifications described above, there is usually no predictive value in classification by target sites or by uses. Such classifications are done, however, to systematically categorize the numerous known poisons. Target sites include the nervous system, the cardiovascular system, the reproductive system, the immune system, and the lungs, liver, and kidneys. Poisons are classified by such uses as pesticides, household products, pharmaceuticals, organic solvents, drugs of abuse, or industrial chemicals.
Transport of chemicals through a cell membrane
In order for a poison to produce toxicity, a sufficient quantity of that chemical must be absorbed into the body. Because the chemical must pass through a number of cell membranes before it can enter the blood, the ability of the chemical to cross these lipid-rich membranes determines whether it will be absorbed, and that ability depends on the chemical's lipid solubility.
The cell membrane, the most external layer of all animal cells, is composed of two layers of lipid molecules (the lipid bilayer). The lipid molecules each have a hydrophilic (water-loving, or polar) end and a hydrophobic (water-hating, or nonpolar) end. Because they are surrounded by an aqueous environment, lipid molecules of the cell membrane arrange themselves so as to expose their hydrophilic ends and protect their hydrophobic ends. Suspended randomly among the lipid molecules are proteins, some of which extend from the exterior surface of the cell membrane to the interior surface.
A chemical tends to dissolve more readily in a solvent of similar polarity. Nonpolar chemicals are considered lipophilic (lipid-loving), and polar chemicals are hydrophilic (water-loving). Lipid-soluble, nonpolar molecules pass readily through the membrane because they dissolve in the hydrophobic, nonpolar portion of the lipid bilayer. Although permeable to water (a polar molecule), the nonpolar lipid bilayer of cell membranes is impermeable to many other polar molecules, such as charged ions or those that contain many polar side chains. Polar molecules pass through lipid membranes via specific transport systems.
The four types of chemical transport systems through cell membranes are diffusion, facilitated diffusion, active transport, and pinocytosis.
As mentioned above, lipophilic, nonpolar chemicals dissolve in the lipid bilayer. Simultaneously, some of the molecules are leaving the lipid bilayer. The net result is that chemicals cross the membrane until the concentrations of chemical molecules on both sides of the membrane are equal and there is no net flow of molecules across the cell membrane ( diffusion). Therefore, chemicals diffuse across the membrane only when a concentration gradient exists across the cell membrane. Diffusion is considered to be passive transport because no external energy is used. Polar molecules, such as water and small water-soluble molecules (e.g., urea, chloride ions, sodium ions, and potassium ions), can diffuse across membranes through the water-filled channels created by membrane proteins. Large polar water-soluble chemicals, such as sugars, however, do not diffuse through the membrane.
Certain relatively large water-soluble molecules cross the cell membrane using carriers. Carriers are membrane proteins that complement the structural features of the molecules transported. They bind to the chemicals in order to move them across the cell membrane. Energy is consumed because the transport proceeds against the concentration gradient.
Active transport systems move chemicals essential to cellular functions through the membrane into the cell. Such essential chemicals include calcium ions, amino acids, carbohydrates, and vitamins. Because the structures of poisons usually are not similar to those of chemicals essential to cells, few poisons are absorbed by active transport. Active transport, however, is important in the elimination of organic acids, bases, and foreign compounds by the kidneys and liver.
Molecules of similar structure compete with one another in binding with the carrier molecule. Thus, the transport of one chemical can be inhibited by another chemical of similar structure, a phenomenon called competitive inhibition. The chemical being transported also competes with itself for a carrier molecule, so that only a limited amount of the chemical can be transported by the carrier protein during a specific time.
Transport systems that use carrier molecules but which do not require energy to proceed are called facilitated diffusion. A chemical first binds to the carrier protein in the cell membrane and then diffuses through the membrane. Because no energy is used, facilitated transport into the cell cannot proceed if the concentration of that chemical is greater inside the cell membrane than outside. The involvement of carriers means that the process is also subject to competitive inhibition and saturation.
Large molecules, such as proteins and solid particles, are often transported by pinocytosis. The cell membrane engulfs a particle or protein molecule outside the cell, and brings it into the cell. Although inefficient, pinocytosis operates in the slow absorption of proteins and particles in the intestine and respiratory tract.
Conditions of exposure
 Figure 1-->
Figure 1--> summarizes the conditions of exposure to toxicants.
summarizes the conditions of exposure to toxicants.Routes of exposure and absorption of chemicals
Injection
Although not a common route of exposure for poisons, injection is the only route in which the entire amount exposed is absorbed regardless of the chemical administered, because the chemical is introduced directly into the body. Chemicals may be injected intravenously (directly into a vein), intramuscularly (into a muscle), subcutaneously (under the skin), and intraperitoneally (within the membrane lining the organs of the abdomen).
Because the blood is the vehicle of chemical distribution in the body, intravenous injection is the most rapid method of introducing a chemical into the body. The almost instantaneous distribution, together with the irreversibility, makes intravenous injection a dangerous method of chemical exposure, with a fair chance of causing drug overdose if improperly administered.
Because there is a relatively large flow of blood to the skeletal muscles, chemicals are absorbed into the blood relatively rapidly after intramuscular injection. The slow absorption of a chemical into the blood after subcutaneous injection is probably due to the low blood flow in the subcutaneous tissues. Intraperitoneal injection is used only in biomedical research. Absorption is relatively rapid with intraperitoneal injection because of the rich blood supply to the abdomen.
Ingestion
Ingestion is the most common route of exposure to toxic chemicals. Most chemicals diffuse across the cell membrane in the nonionized form, so that the degree to which the chemical is ionized (ionization) is important in determining whether a chemical is absorbed (see above Transport of chemicals through a cell membrane (poison)).
Organic acids and bases dissociate into their ionized forms in response to the pH conditions of the environment. Organic acids are in their nonionized form in an acidic environment (such as the stomach), and they thus tend to diffuse across a membrane, whereas organic bases are nonionized and thus diffuse across a membrane in a basic environment (such as in the intestine).
Because the pH and surface areas differ in different segments of the gastrointestinal tract, chemical absorbabilities of these segments also differ. The major sites of absorption of ingested poisons are the stomach and the small intestine, with most of the absorption taking place in the latter. The intestine has a greater blood supply and a much larger surface area. Folds in the mucosa of the small intestine house numerous projections on the luminal surface, which increases the surface area of the 280-centimetre- (110-inch-) long small intestine to up to 2,000,000 square centimetres.
The pH on the mucosal surface of the small intestine is alkaline. Organic bases tend to be in the nonionized, lipid-soluble form and thus in general are absorbed there. The pH of the stomach contents is in the range of 1 to 2 (strongly acidic), and weak organic acids tend to be in the nonionized, lipid-soluble form. It might be expected that the poisons would be absorbed there, but, because the surface area of the stomach is much smaller than that of the small intestine, often the stomach contents (along with the poisons) are passed to the intestine before the chemicals are absorbed. The acidic environment of the stomach is the main reason for the poor absorption of organic bases by the stomach.
Topical ( skin)
The skin is composed of three layers of tissues—the epidermis, dermis, and subcutaneous tissues—and is an effective barrier to many substances. The outer skin layer is the epidermis, containing five layers of cells. The stratum corneum, which is the outermost epidermal layer, consists of dead cells and is the major barrier to chemical transfer through the skin. Although nonpolar chemicals cross the skin by diffusion through the stratum corneum, no active transport exists in the dead cells of this layer. The second layer, the dermis, is thicker and is composed of loosely packed connective tissue cells in a watery matrix of collagen and elastin fibres, as well as sweat glands, hair follicles, capillaries, and lymphatic vessels. After crossing the epidermis, chemical molecules are absorbed into the circulatory system via the capillaries. The capillaries drain into venules in the subcutaneous tissue.
The stratum corneum is not very permeable to water-soluble molecules and ions, although lipid-soluble molecules do cross it to a certain extent. The permeability is directly proportional to the lipid solubility of the chemical (i.e., highly lipid-soluble chemicals are readily absorbed) and inversely proportional to the molecular weight of the chemical (i.e., the rate of absorption increases as the molecular weight of the molecule decreases).
The rate of percutaneous absorption also varies with the thickness of the stratum corneum at different sites of the body. The rate of absorption is higher for skin on the forehead, axilla, back, and abdomen than for thicker regions like the plantar surface of the foot and the palm. The condition of the skin is also important. Percutaneous absorption is faster when the skin is moist rather than dry.
Solids are not absorbed through the skin because the skin is generally not covered with liquid and because pinocytosis does not operate in dead cells. Liquid chemicals penetrate the skin largely because of their lipid solubility. Gases and certain vapours can be absorbed through the skin also, although to a much lesser extent than via inhalation.
Inhalation
The absorption of inhaled gases and vapours differs from that of aerosols and thus will be discussed separately.
Because the same principles govern the absorption of gases and vapours, the word “gases” is used here to represent both gases and vapours. Absorption of inhaled gases takes place mainly in the lungs. Before the gases reach the lung, however, they pass through the nose, where highly water-soluble, or highly reactive, gas molecules are retained by mucosa.
Unlike intestinal and percutaneous absorption of chemicals, respiratory (respiration, human) absorption of gases does not depend on the pH of the alveoli, because gas molecules are not ionized. It also does not depend on the lipid solubility of the gas molecules, for three reasons. First, the alveolar gas molecules are situated in close proximity to the capillaries. Second, the alveoli form a huge surface for gas absorption. Third, the time it takes for a unit of blood to go through the lungs is more than adequate for gas molecules to diffuse from the alveolar space to the blood.
Gas molecules move into the blood by partitioning, which is a gas-transfer process between two phases, such as between the air and the blood or the blood and the tissues. In partitioning, gas molecules move from a phase of high partial pressure to an adjacent phase of low partial pressure. When an individual first inhales the gas, the partial pressure of the gas is higher in the air than in the blood, driving gas molecules from the alveolar space to the blood. As more gas molecules are driven into the blood, the blood's partial pressure is raised. Eventually the partial pressure gradient between the air and blood dissipates and gas transfer stops; equilibrium is then reached, usually before the blood leaves the lungs.
The blood carries the gas molecules to the rest of the body, where the gas is transferred from the blood to the tissue until equilibrium is reached. The blood picks up more gas molecules in the lungs, and the process continues until the gas in each tissue of the body is in equilibrium with that of the blood entering the tissue. At this time, barring biotransformation, no further net absorption of gas takes place as long as the exposure concentration remains constant. A person can breathe the gas forever and not absorb more, a unique characteristic of gas exposure.
The particle size and water solubility of an aerosol chemical are the important characteristics determining absorption of aerosols. For an aerosol to be absorbed, it must be inhaled and deposited on the respiratory tract. If not deposited, the aerosol particles are exhaled. Aerosols of less than 100 micrometres (0.004 inch) can be inhaled.
The aerosol size also determines the tendency of a particle to be deposited on a certain region of the respiratory tract. The larger aerosols (greater than five micrometres) tend to be deposited in the upper respiratory tract, while the smaller ones (less than five micrometres) have a greater chance of being deposited on deeper sites of the lung. The nose acts as a “scrubber” for larger aerosols and thus protects the lung from injury.
Once deposited, aerosol particles must dissolve in the liquid lining the respiratory tract in order to be absorbed. For most aerosols of poor water solubility, the particles are cleared from the respiratory tract by mechanical or cellular means. In the nasopharyngeal region, mechanical methods of clearance include sneezing and nose blowing for particles deposited on the anterior one-fifth of the nasal cavity. Particles deposited on the remaining portion of the nasal cavity and on the pharynx are removed by tiny hairs, called cilia, on the surface of these two regions, which beat almost continuously to move a covering layer of mucous toward the throat (mucociliary apparatus). Any particles deposited on the mucous are carried along and finally swallowed.
In the tracheobronchial region, mechanical clearance includes coughing and the mucociliary apparatus. The trachea, bronchi, and bronchioles, down to the terminal bronchioles, are covered with mucous and cilia. The mucociliary apparatus moves upward toward the larynx, where the respiratory tract joins the esophagus. The particles are eventually swallowed and may be absorbed by the gastrointestinal tract.
The alveolar region has the slowest rate of particle clearance in the entire respiratory system, unless the particles are water-soluble, in which case they are cleared readily by dissolution. Water-insoluble particles in the respiratory bronchioles and alveoli are removed by cellular means, principally by macrophages—scavenger cells that engulf cellular debris in the body by a process called phagocytosis. Once phagocytosed, macrophages that contain particles are removed by the mucociliary apparatus in the terminal bronchioles. Pinocytosis by the cells lining the alveoli probably move the free particles to the interstitial space, where they either enter the lymphatic capillaries and are carried to the bloodstream, or they undergo a long process of dissolution. It can take years for water-insoluble particles to dissolve, depending on the chemical, which is why water-insoluble particles deposited in the alveolar region tend to remain in the interstitial space for a long time and can cause serious harm.
Frequency of exposure
The second important condition of exposure is frequency: acute (single exposure), subchronic (repeated exposures that in total last for no more than 10 percent of the lifetime of an individual), and chronic (repetitive exposures that last in total longer than 10 percent of the lifetime). The difference between the frequencies of exposure is the length of time a chemical is maintained in a target tissue. A single exposure of a poison at a certain dose may be sufficient to produce a toxic concentration in a target tissue, leading to the development of toxicity. Repetitive exposures at the same dose will then enhance the severity of the injury because of the presence of toxic levels of the chemical in the target tissue. The continuous presence of a toxic amount of poison may impair the ability of the damaged cells to carry out repair and thus prevent any chance of recovery. Consequently, a single dose that produces symptoms and toxic signs can lead to death if repeated over time. Repetitive exposures of some chemicals may also produce a different toxic effect than the acute exposure.

 Toxic accumulation is one of the reasons repetitive exposures of a chemical produce toxicity while a single exposure may not. In a hypothetical case, as depicted in Figure 2-->
Toxic accumulation is one of the reasons repetitive exposures of a chemical produce toxicity while a single exposure may not. In a hypothetical case, as depicted in Figure 2--> , a concentration of more than 100 milligrams per gram in a target tissue is required for chemical A to cause toxic injury. If chemical A is administered at a dose that does not produce toxic levels in the tissue and the elimination of the chemical is essentially complete within 24 hours, repetitive exposures at the same dose once a day will not result in toxicity. With chemical A there will be no difference in toxicity between acute and repetitive exposures. Suppose, however, that there is a similar chemical, B, with a slower elimination rate so that chemical B is not completely eliminated from the target tissue within 24 hours. If the exposure to chemical B is carried out at the same dose as chemical A, the concentration of B in the target tissue will not return to zero after 24 hours. Consequently, daily exposures of B will cause the toxin to accumulate, so that the peak target concentration of B increases daily (Figure 2-->
, a concentration of more than 100 milligrams per gram in a target tissue is required for chemical A to cause toxic injury. If chemical A is administered at a dose that does not produce toxic levels in the tissue and the elimination of the chemical is essentially complete within 24 hours, repetitive exposures at the same dose once a day will not result in toxicity. With chemical A there will be no difference in toxicity between acute and repetitive exposures. Suppose, however, that there is a similar chemical, B, with a slower elimination rate so that chemical B is not completely eliminated from the target tissue within 24 hours. If the exposure to chemical B is carried out at the same dose as chemical A, the concentration of B in the target tissue will not return to zero after 24 hours. Consequently, daily exposures of B will cause the toxin to accumulate, so that the peak target concentration of B increases daily (Figure 2--> ). Eventually, the toxic threshold is reached and injury will develop. Therefore, repetitive exposure can produce toxicity at a dose that does not result in injury if given only once.
). Eventually, the toxic threshold is reached and injury will develop. Therefore, repetitive exposure can produce toxicity at a dose that does not result in injury if given only once.Dose of exposure
The amount of chemical to which a person is exposed is extremely important. The chemical acts at a certain site, called the active site, triggering a biological response in a target tissue. Because the biological effect is caused by the presence of the chemical at the active site, the higher the concentration of the chemical at the site, the greater the response. This is the case with all known poisons, a phenomenon called the dose–response relationship.
The dose–response curve is sigmoid, with the linear portion between approximately 16 percent and 84 percent. To compare the potency of chemicals causing similar responses, the dose that produces a biological response in 50 percent of the subject group is chosen, because it can be calculated with the least chance of error. If the biological response is mortality, the dose that kills 50 percent of the exposed population is known as the lethal dose 50, or LD50. Toxicity ratings for chemicals are based on their LD50s. The toxicity rating indicates the amount of chemical required to produce death, but it should be remembered that all chemicals can kill. Thus, all chemicals are toxic. More important than the toxicity of a chemical is its hazard or risk of usage, a concept that incorporates exposure to dosage. For example, botulinum toxin is not especially hazardous, even though it is supertoxic, because food is well-preserved, keeping the exposure or dose very low. In contrast, ethanol (alcohol) is hazardous even though it is not very toxic, because some people have a tendency to use it to excess.
Distribution of toxicants in the body
Role of the lymphatics
After a chemical crosses the transport barrier at the portal of entry, it remains in the interstitial spaces, the spaces between cells that are filled with water and loose connective tissue. The absorbed chemical can gain entry into the bloodstream directly via the blood capillaries or indirectly via the lymphatic capillaries.
Lymphatic capillaries are minute vessels located in the interstitial spaces, with one end closed and the other end draining into larger lymphatic vessels. Just like blood capillaries, the walls of the lymphatic capillaries are composed of a thin layer of cells, the endothelial cells. Unlike the blood capillaries, however, the junctions between the endothelial cells of the lymphatic capillaries are much looser, and as a result lymphatic capillaries are much more porous than blood capillaries. Plasma proteins and excess fluid in the interstitial spaces from blood capillaries enter the lymphatic capillaries and eventually flow back to the heart via the lymphatic system. Insoluble aerosols that cross the alveolar wall by pinocytosis may be absorbed into the circulatory system after first entering the porous lymphatic capillaries.
Role of the blood
The chemical is distributed via the blood to the various tissues of the body, where the chemical is transported across blood capillary walls. There are four types of blood capillary walls: tight, continuous, fenestrated, and discontinuous.
Tight capillary walls are characterized by tight junctions between the endothelial cells, which prevent the diffusion of large molecules and impede that of hydrophilic molecules. The capillaries in the brain are typical of this type of capillary and form part of the blood–brain barrier.
In a continuous capillary wall, channels about five nanometres wide exist between endothelial cells, allowing most small molecules to pass through. Capillaries of this type are found in the skeletal and smooth muscles, connective tissue, lungs, and fat. Chemicals given by intramuscular or subcutaneous injection are readily absorbed into the bloodstream, as are deposited aerosols that dissolve in the fluid lining the respiratory system and cross the alveolar wall.
In a fenestrated capillary wall, holes as large as 100 nanometres are found in the endothelial cells. Capillaries in the intestine and glomeruli in the kidney have fenestrated capillary walls, which account for the high permeability of blood capillaries for absorption by the intestine and for filtration of the blood by the kidney.
The discontinuous capillary wall, the most porous of all capillaries, contains large gaps between the cells through which large molecules and even blood cells pass. This type of capillary is found in the reticuloendothelial system (including the liver, spleen, and bone marrow), which assists in the removal of aged blood cells.
The porous nature of capillaries in most tissues or organs means that a chemical in the bloodstream can be distributed almost freely to most tissues, except for organs with a barrier. The molecules diffuse from the blood to the interstitial spaces of the tissue and finally into the cells by either diffusion or active transport.
Role of tissue blood flow
The rate at which a chemical accumulates in a particular tissue is influenced by the blood flow to that tissue. The well-perfused organs—i.e., organs that receive a rich blood supply relative to organ weight—include major organs like the liver, brain, and kidney. A middle group receives an intermediate blood supply and includes the skeletal muscle and skin. The poorly perfused group includes the fat and bone. As a chemical is distributed to the tissues by the bloodstream, the chemical concentrations in the well-perfused organs rapidly reach a steady state with the blood concentration while the concentrations of the chemical in the poorly perfused tissue lag behind.
Role of protein binding
The plasma contains many proteins, the most abundant being albumin. Some chemicals are known to bind to albumin. Because albumin is too large to cross the blood capillary wall, chemicals that are bound to this plasma protein are confined in the bloodstream and are not readily distributed to the tissues. Chemicals with a high affinity to bind with plasma proteins have lower concentrations in tissues than do chemicals that are not bound to plasma proteins.
Role of distribution barriers
There are barriers in certain organs that limit the distribution of some molecules. The blood–brain barrier consists of tight capillary walls with glial cells wrapped around the capillaries in the brain. Molecules must diffuse through two barriers to get from blood to the nerve cells of the brain. Despite the barrier, water, most lipid-soluble molecules, oxygen, and carbon dioxide can diffuse through it readily. It is slightly permeable to the ions of electrolytes, such as sodium, potassium, and chloride, but is poorly permeable to large molecules, such as proteins and most water-soluble chemicals. The blood–brain barrier is the reason the ions of some highly water-soluble metals, such as mercury and lead, are nontoxic to the brain of an adult. Children, however, are more sensitive to the toxicity of lead because the blood–brain barrier is less well developed in children.
The second distribution barrier is the blood–testis barrier, which limits the passage of large molecules (like proteins and polysaccharides), medium-sized molecules (like galactose), and some water-soluble molecules from blood into the seminiferous tubules of the testis. Water and very small water-soluble molecules, like urea, however, can pass through the barrier. The lumen of the seminiferous tubules is where sperm cells of more advanced stages develop. It is thought that the barrier protects the sperm cells.
The placental barrier between mother and fetus is the “leakiest” barrier and is a very poor block to chemicals. The placenta is composed of several layers of cells acting as a barrier for the diffusion of substances between the maternal and fetal circulatory systems. Lipid-soluble molecules, however, can cross readily, while the transfer of large-molecular-weight molecules is limited.
Elimination of toxicants
excretion
An organism can minimize the potential damage of absorbed toxins by excreting the chemical or by changing the chemical into a different chemical (biotransformation), or by both methods. The body can excrete exogenous chemicals in the urine, bile, sweat, or milk; the lungs can excrete gases such as carbon monoxide.
Urinary excretion, the most common excretory pathway, takes place in the kidney, where the functional units are the glomerulus (a filter) and the renal tubule. The artery entering the glomerulus divides into capillaries, with fenestrated walls encased in the Bowman's capsule. Twenty percent of the blood is filtered through the holes in the capillary walls; molecules smaller than 60,000 molecular weight end up in the filtrate, while red blood cells, large proteins, and chemicals bound to plasma proteins are not filtered.
Chemical exchange can also take place along the renal tubule. As the filtrate flows down the renal tubule, essential molecules, such as amino acids and glucose, are reabsorbed by active transport in the first portion of the tubule (the proximal tubule). Chemicals in the filtrate are also reabsorbed by active transport if they structurally resemble these essential molecules. Unlike glomerular filtration, tubular resorption of a chemical is not influenced by whether or not it is bound to plasma proteins.
As the fluid flows down the renal tubule, water and some chemicals are reabsorbed from the tubular fluid into the blood by diffusion. The tubular fluid emerges from the kidney and is collected in the urinary bladder. Lipid-soluble chemicals are readily reabsorbed in the renal tubule, and only water-soluble chemicals are excreted in the urine to a significant extent.
The second major excretory route is the bile, which is formed in the liver and flows into the intestinal tract. The liver does not filter chemicals as does the kidney, but the liver does secrete chemicals into bile. Chemicals excreted in the bile are eventually eliminated in the feces.
Biliary excretion of a chemical does not necessarily result in the elimination of the chemical from the body. Bile is dumped into the small intestine; there is a chance that chemicals in the bile may be reabsorbed by the intestine and in turn reenter the liver via the portal vein. This cycling of a chemical, known as the enterohepatic cycle, can continue for a long time, keeping the chemical in the body.
During inhalation exposure, absorption of the gas continues until the partial pressure of the gas in the tissues is equal to that of the inspired gases in the lungs. As soon as the concentration of inspired gases decreases or the exposure terminates, respiratory (respiration, human) excretion of the gas occurs. Because the partial pressure of the inspired gas is lower in the lungs than in blood, the blood releases some gas molecules into the alveolar space and these molecules are exhaled. The tissues lose gas molecules to the blood, which carries them to the lungs to be excreted.
The composition of sweat is similar to that of plasma except that sweat does not contain proteins. After secretion, the fluid moves through the sweat duct, where salt and water are reabsorbed. The exact mechanism of sweat secretion is not known. It appears that sweat is a filtrate of plasma that contains electrolytes (such as potassium, sodium, and chloride) and metabolic wastes (like urea and lactic acid). Because sweat resembles a filtrate of plasma, water-soluble chemicals, like some drugs and metal ions, are found in sweat. Sweat is not a major route of excretion of chemicals, however.
milk is a potential, albeit minor, route of chemical excretion, but more importantly it is a potential means of chemical exposure for breast-fed infants.
Most chemicals enter milk by diffusion. Therefore, only the nonionized, lipid-soluble (lipid) forms of organic chemicals are found to a significant extent in milk. Chemicals with a molecular weight less than 200 and that are present in plasma not bound to proteins are more likely to be found in milk. Because the lipid content of milk is higher than that of plasma, highly lipid-soluble chemicals can exist in a more concentrated level in milk than in plasma. Therefore, milk can be a significant route of excretion for highly lipid-soluble chemicals in lactating women.
Biotransformation
Biotransformation, sometimes referred to as metabolism, is the structural modification of a chemical by enzymes in the body. Chemicals are biotransformed in several organs, including the liver, kidneys, lungs, skin, intestines, and placenta, with the liver being the most important. Chemicals absorbed in the gastrointestinal tract must pass through the liver, where they can be biotransformed and thus eliminated before being distributed to other parts of the body. This phenomenon is known as the first-pass effect. As a result, smaller amounts of certain chemicals are distributed throughout the body after oral administration than after other exposure routes, such as intravenous or intramuscular injections. Biotransformation of a chemical primarily facilitates its excretion into urine or bile; however, certain chemicals are biotransformed into more toxic forms and, as a result, biotransformation of chemicals is not always beneficial.
Biotransformation of exogenous chemicals (chemicals that are not naturally found in the body) generally occurs in two phases. In phase I, an exogenous molecule is modified by the addition of a functional group such as a hydroxyl, a carboxyl, or a sulfhydryl. This modification allows phase II, the conjugation, or joining, of the exogenous molecule with an endogenous molecule (one naturally found in the body), to take place. The major end product in most cases is a more water-soluble chemical that is easily excreted.
Phase I reactions can be classified as oxidation, reduction, or hydrolysis. Oxidation is carried out by cytochrome P-450 monooxygenases, mixed-function amine oxidases, and alcohol and aldehyde dehydrogenases. The reactions mediated by cytochrome P-450 monooxygenases can make the chemical less toxic or more toxic. The cytochrome P-450 enzymes can, for example, produce epoxides of some chemicals, which are very reactive and can attack important cellular molecules, such as DNA. The remaining phase I oxidative enzymes act on a narrow range of substrates.
In addition to the oxidation of a chemical, cytochrome P-450 monooxygenases can catalyze the reduction. Another group of enzymes that can carry out reduction is the aldehyde/ketone reductases. Each of the three groups of hydrolytic enzymes (epoxide hydrolases, esterases, and amidases, respectively) creates metabolites with a hydroxyl, carboxyl, or amino functional group.
In phase II reactions an altered exogenous chemical binds with an endogenous molecule, leading to the formation of a final product (the conjugate), which is usually much more water-soluble and easily excreted than the parent chemical. There are four types of parent compounds whose excretion can be enhanced by conjugation: glucuronic acid, glutathione, amino acids, or sulfate. The first two types are the most common phase II reactions.
Conjugation of glucuronic acid with a hydroxyl, carboxyl, amino, or sulfhydryl group leads to the formation of oxygen, nitrogen, or sulfur glucuronides, which are more easily excreted than glucuronic acid because they are more water soluble and because they contain a carboxyl group. Conjugation with glutathione also enhances excretion. Glutathione conjugation yields glutathione conjugates and mercapturic acid derivatives, which are excreted by the liver, kidney, or both.
Two types of conjugations, acetylations and methylation, do not enhance the excretion of the parent chemical. Acetylation and methylation decrease the water solubility of the parent chemical and mask the functional group of the parent chemical, preventing these functional groups from participating in conjugations that increase their excretion. Acetylation acts on chemicals with an amino group and may render them less toxic. Chemicals with an amino, hydroxyl, or sulfhydryl group can be methylated. Methylation is not as important a route of biotransformation for exogenous chemicals as it is for endogenous chemicals.
Therapeutic, toxic, and lethal responses
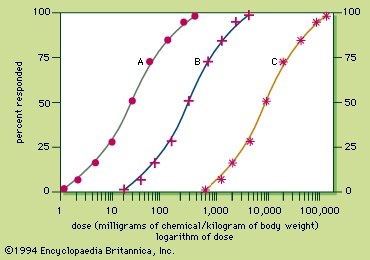 Because the response to a chemical varies with the dose, any substance can be a poison. Medicine can produce responses that are therapeutic (beneficial) or toxic (adverse), or even lethal. The sigmoid dose–response relationships for the therapeutic and lethal responses typically look like curves A and C, respectively, of Figure 3-->
Because the response to a chemical varies with the dose, any substance can be a poison. Medicine can produce responses that are therapeutic (beneficial) or toxic (adverse), or even lethal. The sigmoid dose–response relationships for the therapeutic and lethal responses typically look like curves A and C, respectively, of Figure 3--> . If drug X has therapeutic, toxic, and lethal dose–response curves of A, B, and C, respectively, X is a very safe drug, since there is no overlap of the curves. For some medicinal agents, there is overlap of the therapeutic and lethal dose-response curves, so that a dose which causes a therapeutic response in some individuals can kill others. These agents, consequently, are not as safe.
. If drug X has therapeutic, toxic, and lethal dose–response curves of A, B, and C, respectively, X is a very safe drug, since there is no overlap of the curves. For some medicinal agents, there is overlap of the therapeutic and lethal dose-response curves, so that a dose which causes a therapeutic response in some individuals can kill others. These agents, consequently, are not as safe.A quantitative measurement of the relative safety of drugs is the therapeutic index, which is the ratio of the dose that elicits a lethal response in 50 percent of treated individuals (LD50) divided by the dose that elicits a therapeutic response in 50 percent of the treated individuals (TD50). For instance, the therapeutic index of drug X is 9,000 milligrams per kilogram divided by 30 milligrams per kilogram and is equal to 300. The larger the therapeutic index, the safer the drug. Diazepam and digoxin are examples of drugs with a large and a small therapeutic index, respectively.
Morphological versus functional toxic responses
Chemicals can elicit various types of toxic responses, which can be classified by the nature of the response, the site of toxic action, the time it takes for the response to develop, and the chance of resolution of the response. The nature of the toxic response can be morphological (structural) or functional or both. In most cases, the chemical produces morphological changes in an organ, which in turn affects the function of the organ. In a small number of cases, the chemical produces functional changes in an organ without changing the structure of the organ.
Inhalation exposures to silica dust at a low concentration for 10 years or more can lead to chronic silicosis, a condition characterized by the formation in the lungs of silicotic nodules, which are egg-shaped lesions composed of layers of fibroblasts (reparative cells) and inflammatory cells surrounding a central silica particle. Such lesions can be considered a morphological toxic response; unless the silica exposure is prolonged, there will be little respiratory impairment because the lungs and certain other organs have a large functional reserve. If the silica exposure is prolonged, however, the silicotic nodules coalesce (complicated silicosis), and the structure of the lungs is altered so drastically that they do not distend easily during inspiration. Oxygen exchange in the alveoli is impaired, causing such functional toxic responses as breathlessness, chest tightness, and coughing with sputum.
Malathion exposure, on the other hand, can lead to functional toxic responses without causing any morphological changes. Malathion does not alter the structure of tissues; rather, it inhibits an enzyme, acetylcholinesterase, which normally degrades acetylcholine, the neurotransmitter of the parasympathetic nervous system. Inhibition of this enzyme leads to an exaggeration of the actions of the parasympathetic nervous system, including sweating, secretion of saliva, adjustment of pupil size, and defecation. The end results are increased perspiration, increased salivation, tearing, blurred vision, abdominal cramping, diarrhea, and if severe enough, death from respiratory depression.
Local versus systemic toxic responses
Toxic responses are also classified according to the site at which the response is produced. The site of toxic response can be local (at the site of first contact or portal of entry of the chemical) or systemic (produced in a tissue other than at the point of contact or portal of entry).
An example of a local toxic effect is the tissue corrosion produced by strong acids (e.g., sulfuric acid) and bases (e.g., sodium hydroxide) in contact with tissues. If the exposure is external, skin burns result; if ingested, the acid or base causes serious local damage to the esophagus and stomach.
An example of a systemic toxicant is methanol, which is absorbed and biotransformed into formic acid. The acid is responsible for metabolic acidosis and optic nerve damage in the retina of the eye, leading to visual impairment, a systemic effect.
Immediate versus delayed toxic responses
Toxic responses may also be classified according to the time it takes for development of a toxic response. If it takes up to a few days after exposure, the response is considered immediate. There is no universal standard of minimum time for delayed toxic responses, but generally a response that takes more than a few days to develop is considered delayed. The time it takes for a systemic toxicant to act depends on many factors, such as the rates of absorption, biotransformation, distribution, and excretion, as well as the speed of action at the target site.
Reversible versus irreversible toxic responses
Toxic responses differ in their eventual outcomes; the body can recover from some toxic responses, while others are irreversible. Irritation of the upper respiratory tract by inhaled formaldehyde gas, for example, is rapidly reversible in that as soon as the inhalation exposure terminates, the irritation subsides. In contrast, the response produced by silica dust is irreversible because, once the silicotic nodules are formed, they remain in the alveolar region of the lung.
Chemically induced immune responses
The immune system protects the body against foreign substances, especially microbes and viruses. To be antigenic, a substance is usually both relatively large and foreign to the body. Large proteins are often strong antigens. Smaller chemicals can become antigenic by combining with proteins in chemicals called haptens.
Cellular and humoral immunities
The development of immunity toward an antigen is called sensitization. After exposure to an antigen, a combination of cellular and humoral immunity usually develops. Exposure routes that favour slow absorption into the bloodstream, such as percutaneous injection, often primarily elicit cellular immunity, while rapid routes of exposure, such as intravenous injection, favour the development of humoral immunity.
Cellular immunity utilizes phagocytes (such as macrophages, neutrophils, and eosinophils), which engulf antigens, and T-lymphocytes, which are thymus-derived, antigen-specific immune cells containing receptors specific for a special antigen. Cellular immunity is particularly important in defending the body against tumours and infections. Macrophages phagocytize antigens and secrete proteins (monokines) that regulate cells involved in immune responses. One monokine is interleukin-2, which stimulates an increase in the number of T-lymphocytes. The T-lymphocytes then develop surface receptors for specific antigens. Because T-lymphocytes survive for months or years, cellular immunity toward the antigen remains with the individual for a long time. If reexposed to the same antigen, the sensitized T-lymphocytes recognize the antigen and secrete their own proteins (lymphokines), which stimulate phagocytes to destroy the antigen. If an antigen is located on foreign or tumour cells, certain T-lymphocytes are transformed into cytotoxic T-lymphocytes, which destroy the target cells.
Humoral immunity utilizes antibodies, also known as immunoglobulins (antibody) (Ig), produced by B-lymphocytes. B-lymphocytes are lymphocytes derived from the spleen, tonsils, and other lymphoid tissues. They become plasma cells, which make antibodies. There are five classes of antibodies: IgG, IgM, IgA, IgD, and IgE. IgG, IgM, and IgA are involved in humoral immunity, the function of IgD is not known, and IgE takes part in immediate hypersensitivity (see below).
Humoral immunity involves the inactivation, removal, or destruction of antigens. Antibodies can inactivate viruses by binding to them. With two antigen binding sites per protein unit, an antibody can also precipitate the antigen by cross-linking in a network formed with other antibodies. Because each IgM has five protein units, and thus five potential binding sites, IgM is particularly efficient in precipitating the antigen. After the antigen is precipitated, it can be removed by phagocytes. In addition, antigen binding by IgG or IgM activates a serum protein, called a complement, which can then initiate antigen precipitation, amplifying the inflammatory response. If the antigen is on the surface of certain cells, activated complement can also facilitate the lysis of these cells. IgG or IgM can also link the antigen to phagocytes or to killer cells, resulting in lysis of the cell by an unknown mechanism.
Allergies
Although the immune system generally protects the body, it can respond in certain ways that are detrimental to some individuals. Allergy, or hypersensitivity, is a condition of increased reactivity of the immune system toward an antigen that leads to adverse effects. Substances that cause allergies are known as allergens.
Confusion is sometimes caused by the terms hypersensitivity (allergy), hypersusceptibility, and idiosyncrasy. Hypersensitivity is a reaction to a chemical or substance in certain individuals and has a basis in the immune system. Hypersusceptibility is an increased predisposition of certain individuals to react to a chemical. Because of biological variability among humans, some individuals respond to a chemical at a dose too low to produce a similar effect in others. Idiosyncrasy is a genetically determined hypersusceptibility.
Allergic responses differ from the usual toxic responses in three ways. First, the allergic response does not occur during the first exposure to an allergen, but is evident only after at least one previous exposure. In rare occasions, an allergic response can occur on the first exposure to a chemical if the individual has already developed a hypersensitivity toward a closely related chemical. For example, people allergic to one kind of penicillin are usually allergic to other penicillins as well. Second, allergy is specific to both the allergen and the individual. Unlike in a toxic response, in which everyone exposed develops the response if a sufficient dose is administered, only a small fraction of the exposed population is sensitized by an allergen, regardless of the dose. Third, the amount of a chemical required to elicit an allergic response is usually much less than that required to produce a toxic response.
There are four types of hypersensitivities (allergies): immediate, cytotoxic, immune-complex, and delayed. Each differs from the others in the mechanism of induction and the responses produced. Immediate hypersensitivity is the most common form of allergy. Delayed hypersensitivity is the second most common, whereas cytotoxic and immune-complex hypersensitivities are relatively rare.
Immediate hypersensitivity, also called anaphylaxis (atopy), produces IgE in response to an allergen that binds to the surface of mast cells or basophils. When reexposed to the allergen, the antigen-binding end of IgE on mast cells and basophils binds the allergen, triggering a release of anaphylactic mediators from these cells. These mediators, such as histamine and serotonin, cause the contraction of certain smooth muscles (e.g., those of the respiratory tract, leading to bronchoconstriction in asthmatic attacks), relaxation of blood vessels (e.g., in the skin, resulting in redness, or in the whole body, causing a fall in blood pressure as in anaphylactic shock), and increased permeability of capillary walls (e.g., in the skin, leading to local edema as seen in urticaria). The unique characteristic of immediate hypersensitivity is its rapid onset, with the response initiated within a few minutes of allergen exposure.
The anaphylactic mediators affect tissues differently. Thus, the allergic response depends on where the immune reaction takes place. In the skin, immediate hypersensitivity can result in skin eruptions or urticaria, characterized by wheals with redness. In the respiratory system, it can produce hay fever or asthma. In the gastrointestinal tract, allergic gastroenteritis, an inflammatory condition of the stomach and intestine, may result. Systemic anaphylaxis may involve the entire body, with shock as a key feature.
A second type of hypersensitivity is cytotoxic hypersensitivity, which has a gradual onset. After reexposure to an allergen, the allergen molecules attach to the surfaces of blood cells, forming an antigen new to the body. IgG or IgM binds to the new antigen on the blood cells, lysing blood cells via either complement fixation or antibody-dependent cell cytotoxicity. If the lysed cells are red blood cells, hemolytic anemia results. If platelets (the blood components intrinsic to blood clotting) are lysed, however, the blood clotting mechanism is impaired.
In a third type of allergy, immune-complex hypersensitivity, the allergen-IgG complex precipitates in tissues, resulting in inflammation via complement fixation. Immune-complex hypersensitivity in the kidney results in an inflammatory injury of the glomeruli (glomerulonephritis), and in the lung it leads to a pneumonia-like condition known as hypersensitivity pneumonitis.
Delayed hypersensitivity differs from other types in not involving humoral immunity. Upon reexposure to the allergen, sensitized T-lymphocytes release lymphokines, which trigger a series of inflammatory reactions. The inflammation leads to the development of allergic contact dermatitis in the skin and a chronic form of hypersensitivity pneumonitis in the lung. Symptoms of allergic contact dermatitis develop gradually, taking a day or two to reach maximum levels, which is the best way to distinguish allergic contact dermatitis from atopic dermatitis with similar symptoms. In contrast, the chronic form of hypersensitivity pneumonitis develops insidiously and not in a fixed time.
Teratogenesis
Teratogenesis is a prenatal toxicity characterized by structural or functional defects in the developing embryo or fetus. It also includes intrauterine growth retardation, death of the embryo or fetus, and transplacental carcinogenesis (in which chemical exposure of the mother initiates cancer development in the embryo or fetus, resulting in cancer in the progeny after birth).
Intrauterine human development has three stages: implantation, postimplantation, and fetal development. The first two stages are the embryonic stages and last through the first eight weeks after conception. The fetal stage begins in the ninth week and continues to birth.
Depending on the developmental stage, chemical exposure in the mother can result in different degrees of toxicity in the embryo or fetus. In the preimplantation period, a toxic chemical can kill some of the cells in the blastocyst, resulting in the death of the embryo. During the postimplantation period, chemical-induced cell death leads to one of two outcomes. If death is confined to those cells undergoing active cell division at the moment, the corresponding organs are affected, resulting in malformation. If the cell death is generalized without significant replication by the remaining cells to sustain life, the embryo dies. During the third, fetal, period, chemical injury can retard growth or, if severe enough, kill the fetus.
The genesis of a particular organ ( organogenesis) occurs at a specific time during gestation and is not repeated. Because organogenesis is a tightly programmed sequence of events, each organ system has a critical period during which it is sensitive to chemical injury. Chemical exposure in a critical period is likely to produce malformations of that organ and not others; however, because there is some overlapping of critical periods of organ development and because chemicals frequently remain in the embryo for a period of time, malformations of more than one organ usually occur. Since organogenesis occurs mostly in the embryonic stages, chemical exposure in the first trimester should be minimized, if possible.
Little is known about mechanisms of teratogenesis. It is thought that some teratogens produce malformations directly by killing the cells in the embryo. Teratogens can also produce malformations indirectly by causing maternal toxicity, resulting in oxygen or nutrient deficiency for the embryo. A few well-known examples are discussed below.
thalidomide is a drug originally marketed to combat nausea and vomiting in pregnancy. It was discovered in the 1960s in West Germany to cause rare limb defects, among other congenital anomalies. The discoveries about thalidomide triggered legislation requiring teratogenicity (mercury poisoning) testing for drugs.
Chronic alcohol (alcohol consumption) ingestion during pregnancy is the most common cause of congenital problems in mental development. Ingestion of more than 30 millilitres (1 ounce) of ethyl alcohol per day during pregnancy can lead to the development of fetal alcohol syndrome, characterized by intrauterine growth retardation and subsequent learning disabilities, such as distractibility, language disorders, and low IQ. Heavier consumption of alcohol, more than 60 millilitres per day, by a pregnant woman can result in malformations of the fetal brain and in spontaneous abortions.
diethylstilbestrol (DES) is a drug used primarily from the 1940s to the '50s to prevent miscarriage. The drug is an example of a chemical that can produce transplacental carcinogenesis. It was discovered in the early 1970s that exposures to diethylstilbestrol before the ninth week of gestation could lead to the formation of rare vaginal and cervical cancers in female progenies.
Carcinogenesis (carcinogen)
Carcinogens are chemicals that can produce tumours, abnormal tissue growths caused by a loss of control in cell replication. Most tumours are solid masses (e.g., lung cancer), but some do not occur as tissue swellings (e.g., leukemia).
Tumours may be benign or malignant. Benign tumours are to a certain degree controlled in their growth. As a result, benign tumours maintain some form of cellular organization and grow rather slowly over a period of years. In contrast, cell growth in malignant tumours is almost totally uncontrolled. Cells in malignant tumours grow very rapidly in a disoriented fashion.
Benign tumours are encapsulated by a fibrous layer and so do not invade surrounding tissue. Malignant tumours invade surrounding tissue. Thus, while a benign tumour grows at one site, a malignant tumour sends out cancerous cells via the blood and lymphatic system to distant sites of the body, spreading by a process known as metastasis. The invasion of surrounding tissues by a malignant tumour produces various symptoms.
Carcinogenesis is a complicated process in which many factors are known to play significant roles. Certain external environmental factors are important. For instance, cigarette smoking is known to cause predisposition to the development of lung cancer. A diet low in fibre content and high in fat is correlated with a high incidence of colorectal cancer. In addition, internal factors, such as hormonal imbalances and immunosuppression, can also increase the chance of developing tumours. Sensitivity to chemical carcinogens is known to be species-dependent. A chemical carcinogen may induce tumours in one animal species but not another, and a species that is sensitive to one carcinogen may be resistant to another. Known human carcinogens include some anticancer drugs, aromatic (containing a benzene ring in its chemical structure) amino and nitro compounds, metals, radionuclides, and miscellaneous chemicals. In humans the respiratory tract is the most common target for chemical carcinogens, followed by the liver and the blood.
Although there have been many theories on the mechanism of chemically induced tumour formation, it is now thought that DNA is the target of most chemical carcinogens. The carcinogens interact with the DNA and interfere with its normal function. Because DNA controls cellular functions, when DNA is damaged, the cell presumably loses control and divides in a chaotic fashion. A clone of the parent cell is generated, and these cells maintain the chaotic replication, which ultimately leads to the formation of a tumour. In general it takes 10 to 20 years for the initial DNA damage in one cell to develop into a recognizable tumour.
Carcinogens that are thought to produce cancer in laboratory animals by altering the DNA are referred to as genotoxic carcinogens. They are either direct-acting or indirect-acting chemicals.
Direct-acting (reactive) genotoxic chemicals can themselves interact with DNA. Indirect-acting genotoxic carcinogens do not bind to DNA until they have been biotransformed in the body to reactive chemicals. Among the indirect-acting carcinogens, polycyclic aromatic hydrocarbons, nitrosamines, and nitrosonornicotine are found in cigarette smoke. Polycyclic aromatic hydrocarbons are also formed in charcoal-broiled meat. Nitrosamines can be formed by the nitrosation of nitrite-cured, protein-rich food, such as nitrite-cured meat and fish, in the intestine.
Chemicals that produce cancer by a mechanism other than by binding to DNA are known as epigenetic carcinogens. The mechanisms by which epigenetic carcinogens produce tumours are not known with certainty, but various theories have been proposed. Cytotoxins are thought to kill cells in the target organ. The cell death increases cell replication by the remaining cells, which somehow results in tumour development, possibly by stimulating the division of cells that have previously had their DNA damaged by a genotoxic carcinogen.
It has been proposed that hormones and chemicals which modify the activities of the endocrine system create a physiological imbalance in organs dependent for their functioning on a particular hormone. With the imbalance, the organ may lose its normal physiological control and tumour growth may occur. This may be the mechanism by which estrogens in postmenopausal women lead to development of uterine cancer and the reason antithyroid agents, such as 3-amniotriazole, produce thyroid tumours.
Chemicals that depress the immune system are thought to produce tumours by impairing cell-mediated immunity, which is important in the normal elimination of tumour cells. The development of tumours involves two main steps: initiation and promotion. Initiation is the creation by genotoxic carcinogens of a cell with abnormal DNA. After initiation, promoters stimulate the replication of these neoplastic cells and facilitate the development of the tumour. Initiators include genotoxic chemicals. Although promoters do not produce tumours directly, they are still considered carcinogens because they can lead to the development of tumours in concert with an initiator. Promoters include large chlorinated hydrocarbon molecules (e.g., DDT, PCBs, TCDD, butylated hydroxy antioxidants, and saccharin) and tetradecanoyl phorbol acetate in croton oil.
Mutagenesis
Mutagenesis is the alteration of genes. Substances able to produce mutations are naturally genotoxic substances. Once a gene is mutated in a cell, the altered gene can be passed on to daughter cells. The body has ways to repair some of these gene alterations so that the genetic damage does not always propagate.
The effect that a mutation has depends on the cell in which the mutation occurs. In the somatic cells of most organs, mutation either has no effect, causes one cell to die, or causes a cell to divide at an uncontrolled rate so that a tumour develops. If the mutation occurs in germ cells (egg and sperms), there may be detectable changes or birth defects, or stillbirth may result.
Types of poison
In regard to poisoning, chemicals can be divided into three broad groups: agricultural and industrial chemicals, drugs and health care products, and biological poisons—i.e., plant and animal sources. These three groups, along with a fourth category, radiation, are discussed below.
Agricultural and industrial chemicals
Agricultural chemicals
The majority of agricultural chemicals are pesticides, which include insecticides (insecticide), herbicides, fungicides, fumigants, and rodenticides.
Insecticides
Agricultural chemicalsThe four main classes of insecticides are organophosphates, carbamates, chlorinated hydrocarbons, and insecticides derived from plants (botanical). Organophosphate and carbamate insecticides act by inhibiting acetylcholinesterase, the enzyme that degrades acetylcholine (the messenger of the parasympathetic nervous system). As a result, acetylcholine levels remain high, exaggerating the normal functions of the parasympathetic system (Table 1 (Agricultural chemicals)). Effects such as salivation, lacrimation, urination, defecation, twitching of the skeletal muscles, and in severe poisoning, death from respiratory depression occur.
Agricultural chemicalsChlorinated hydrocarbons used as insecticides, such as chlorophenothane ( DDT), are larger molecules than the chlorinated hydrocarbons used as organic solvents, such as chloroform. The former stimulate the central nervous system; the latter depress it. The major toxic effect produced by these insecticides is convulsions (Table 1 (Agricultural chemicals)). The use of DDT is banned in many countries because of its environmental effects and because it may cause cancer in humans. DDT is a highly fat-soluble chemical that accumulates in fish, and, when birds eat such fish, the chemical also accumulates in their fat tissues. The DDT in the birds results in fragile eggs, which are prone to breakage. This will ultimately decrease the population of fish-eating birds.
Agricultural chemicalsIn general, insecticides derived from plants are low in toxicity. Pyrethrins are widely used insecticides in the home. They have a rapid “knockdown” for insects and have a low potential for producing toxicity in humans. The major toxicity of pyrethrins is allergy. Rotenone is a mild irritant and animal carcinogen (Table 1 (Agricultural chemicals)).
Herbicides (herbicide)
Agricultural chemicalsHerbicides are chemicals used to kill plants. Their potential to produce toxicity in humans is rather low. High doses of 2,4-D, however, can produce muscular and neurological symptoms (Table 1 (Agricultural chemicals)). The systemic toxicity of 2,4,5-T is lower than that of 2,4-D, but 2,4,5-T is more irritating.
During the Vietnam War, Agent Orange, a mixture of 2,4-D and 2,4,5-T, was used as a defoliant. The 2,4,5-T used in the Agent Orange was contaminated with tetrachlorodibenzodioxin (TCDD), or dioxin. Although TCDD is extremely toxic to some animals, it is less so to others, but it does cause birth defects and cancer in laboratory animals. The major toxicity of TCDD in humans is in the production of chloracne, a condition characterized by acne that appears between the eyes and the ears. In more severe form, acne may be found on the face, trunk, and buttocks. (Significant adverse health effects in the soldiers exposed to low amounts of TCDD in Vietnam have not been clearly established.) Polychlorinated biphenyls (PCBs) also produce chloracne by damaging the sebaceous glands in skin.
Rodenticides
Agricultural chemicalsWarfarin was originally developed as a drug to treat thromboembolism, a disease caused by blood clots, since it inhibits the synthesis of a factor essential for the clotting of blood. The inhibition of blood clotting by warfarin can lead to internal bleeding (Table 1 (Agricultural chemicals)), however. Because of its ability to induce internal bleeding, warfarin is also used as a rodenticide.
Plant growth regulator
Agricultural chemicalsDaminozide, also known as Alar, is a plant growth regulator used to improve the appearance and shelf life of apples. Because of its carcinogenicity in animals (Table 1 (Agricultural chemicals)), concerns have been raised that daminozide may produce tumours in children who consume apples. As a result, the use of daminozide has greatly decreased.
Industrial chemicals
The term industrial chemicals is used to refer to chemicals used neither in agriculture nor as drugs. Therefore, it includes chemicals used in industry, as well as chemicals found in or near households. Poisoning with industrial chemicals occurs most often by either percutaneous or inhalation routes.
Organic compounds
Industrial chemicalsDepression of the central nervous system is a common effect of most hydrocarbons (hydrocarbon) (Table 2 (Industrial chemicals)). Examples of common hydrocarbons include gasoline, toluene, and heptanes; n-hexane; and benzene. The hydrocarbons are lipid-soluble and dissolve in the membrane of nerve cells in the brain, perturbing their function. Depression, such as drowsiness, occurs as a result. In addition, many of the hydrocarbons sensitize the heart to fibrillation by epinephrine. The hydrocarbon n-hexane also causes damage to peripheral nerves. Benzene is toxic to organs like the bone marrow that form blood cells and can lead to the production of leukemia.
Industrial chemicalsMost alcohols (alcohol consumption) produce depression of the central nervous system, but some alcohols cause certain unique toxicities. Examples of common alcohols include methanol, ethanol, isopropanol, ethylene glycol, and phenol. Methanol can produce blindness after being metabolized to formic acid, which also leads to acidosis, characterized by an acidic pH in the body (lower than the normal pH of 7.4). Ethanol (ethyl alcohol) produces birth defects in both laboratory animals and humans. It also produces fetal alcohol syndrome, a major cause of mental retardation, in children of mothers who drink excessively while pregnant. Ethanol is toxic to the liver in chronic alcoholism and is a major cause of cirrhosis, a condition characterized by hardening of the liver. Phenol differs from other alcohols in causing damage to multiple organs. Finally, ethylene glycol, which is widely used as an antifreeze agent in automobiles, causes renal damage when it is biotransformed to oxalic acid, which crystallizes in the renal tubule (Table 2 (Industrial chemicals)).
Industrial chemicals Industrial chemicalsThe major toxicity produced by aldehydes (aldehyde), such as formaldehyde, is irritation (Table 2 (Industrial chemicals)). Formaldehyde can also cause allergic reactions in people who have been sensitized to it. Examples of other common aldehydes include acetaldehyde, glutaraldehyde, and acrolein. The toxicities of ketones (ketone) and esters (ester) are similar to those of aldehydes in causing mainly irritation of the respiratory tract if inhaled and the gastrointestinal tract if ingested. (Table 2 (Industrial chemicals)).
Industrial chemicalsAromatic amines (amine) and nitro compounds, for example, aniline, toluidine, and nitrobenzene, produce depression of the central nervous system and methemoglobinemia (Table 2 (Industrial chemicals)). Methemoglobinemia is a condition in which the ferrous ion in hemoglobin, which is responsible for carrying oxygen, is oxidized to the ferric form. Oxidized hemoglobin, called methemoglobin, can still carry oxygen, but it does not readily release oxygen to tissues, so that the body, in effect, has a lack of oxygen. Some aromatic amines and nitro groups are known to cause bladder cancer.
Industrial chemicalsBecause both anhydrides (anhydride) and isocyanates are highly reactive, they are extremely irritating to the upper respiratory tract (Table 2 (Industrial chemicals)). If the airborne concentration is sufficiently high, the upper respiratory tract cannot remove all of the isocyanate or anhydride molecules, and pulmonary injury (mainly edema) results. Such a situation occurred in Bhopal, India, in the mid-1980s, when methyl isocyanate from a chemical plant was inadvertently released into the air, killing as many as 2,500 people and injuring thousands of others. Because they are chemically reactive, anhydrides and isocyanates also tend to cause hypersensitivity responses, such as asthma and allergic contact dermatitis. Common examples of anhydrides include maleic anhydride and phthalic anhydride; examples of isocyanates include methyl isocyanate and toluene diisocyanate.
Industrial chemicalsMiscellaneous organic chemicals include such compounds as phosgene, carbon disulfide, and the halogenated aromatic compounds. Phosgene gained notoriety when it was used in chemical warfare in World War I. Like anhydrides and isocyanates, phosgene is highly reactive. Instead of reacting with the mucosal linings of the upper respiratory tract, however, it tends to react with the lungs, causing edema. As a result, the lungs' defenses against bacteria are weakened, and pneumonia may occur. Halogenated aromatic compounds with more than one ring, such as polychlorinated biphenyls (polychlorinated biphenyl) (PCBs), polybrominated biphenyls (PBBs), and 2,3,7,8-tetrachlorodibenzodioxin TCDD, can produce a number of toxic effects in laboratory animals, including cancer, birth defects, liver injury, porphyria, and immunotoxicity (Table 2 (Industrial chemicals)). The PCBs have been extensively used as a cooling agent in electrical transformers. It appears that humans are more resistant to the toxicity of these compounds than are some species of laboratory animals, and the main toxic effect observed in humans is chloracne, similar to juvenile acne.
Inorganic compounds
Examples of metal compounds toxic to humans include manganese, lead, cadmium, nickel, and arsenic compounds, beryllium oxide, and the elemental vapours, inorganic salts, and organic compounds of mercury. Chronic manganese exposure can damage the brain, resulting in a condition with symptoms similar to Parkinson's disease, such as slurred speech, masklike face, and rigidity. Mercury can also damage the brain, leading to behavioral changes; however, mercury is also toxic to the peripheral nervous system, causing sensory and motor symptoms. In addition, mercury is toxic to the kidney. Methyl mercury is especially toxic to the developing brain of a fetus.
Industrial chemicals lead is probably the most ubiquitous metal poison. Used for numerous purposes, before World War II it was a major constituent in paint, and it has been used in gasoline. Like mercury, lead is toxic to the nervous system and kidney (Table 2 (Industrial chemicals)), but its toxicity is age-dependent. In children, the blood–brain barrier is not fully developed, and more lead enters the brain. The extent of damage depends on the exposure; at lower levels of exposure, small decreases in intelligence and behavioral changes may result, whereas high levels result in severe brain damage and death. In adults, lead tends to cause paralysis or weakness, indicative of peripheral nervous system damage.
In acute cadmium poisoning by ingestion, irritation of the gastrointestinal tract is the major toxicity, causing nausea, vomiting, diarrhea, and abdominal cramps. With chronic exposure by inhalation, however, kidneys and lungs are the target organs. Arsenic compounds damage many organs. They cause skin lesions, decrease in heart contractility, blood vessel damage, and injuries of the nervous system, kidney, and liver. Arsenic compounds also produce skin and lung tumours in humans. Certain nickel and hexavalent chromium compounds, as well as beryllium oxide, are toxic to the lungs and can cause lung cancer.
Industrial chemicalsAcids (acid), such as sulfuric and hydrochloric acids, and strongly alkaline compounds, such as sodium hydroxide, and potassium hydroxide are corrosive to tissues on contact and can cause severe tissue injuries (Table 2 (Industrial chemicals)). Sulfuric acid, sodium hydroxide, and potassium hydroxide are active ingredients in drain cleaners, the ingestion of which can cause severe chemical burns of the mouth and esophagus.
Industrial chemicalsHypochlorites are often used as bleaching agents. In low concentrations, as in household bleaches, hypochlorites have little toxicity but may be irritating to tissues; they can, however, be corrosive at high concentrations. Cyanide ions poison the oxidative metabolic machinery of cells so that insufficient energy is generated. The effect is as if there were a lack of oxygen for the cells, even though there is plenty of oxygen in the blood. Hydrogen sulfide and chlorine are highly irritating to the respiratory tract, with pulmonary edema the major toxic effect. Chronic fluoride poisoning is called fluorosis, which is characterized by tooth mottling and increased bone density. These changes, especially of the bone, are related to a change in body calcium caused by fluoride. Silica and asbestos remain in the lungs for long periods of time, and both produce lung fibrosis (Table 2 (Industrial chemicals)). In addition, asbestos is a well-known human carcinogen.
General air pollutants
Sulfur dioxide, an acidic pollutant, irritates the respiratory tract. It causes violent coughing when it irritates the throat, and may result in shortness of breath, lung edema, and pneumonia when it reaches the lungs.
Both ozone and nitrogen oxides are oxidizing pollutants. Like sulfur dioxide, they cause respiratory irritation; ozone and nitrogen oxides, however, tend to be more irritating to the lung than to the upper respiratory tract.
carbon monoxide, an asphyxiating pollutant, binds to hemoglobin more strongly than oxygen does. Such binding produces a hemoglobin molecule that cannot carry its normal load of four oxygen molecules. In addition, once carbon monoxide is bound, the hemoglobin molecule does not as readily release to the tissues the oxygen molecules already bound to it. Therefore, tissues lack oxygen, resulting in many toxic effects. Because the brain is especially sensitive to the lack of oxygen, most of the symptoms are neurological. Lack of oxygen is termed asphyxiation, and thus carbon monoxide is an asphyxiant.
Drugs (drug) and health care products
Poisoning with drugs predominantly involves oral exposures. With drugs, therefore, irritation of the respiratory tract is rare, but anorexia, nausea, and vomiting resulting from gastrointestinal irritation are common.
Painkillers
Drugs and health care productsPainkillers (analgesics) are the most commonly used drugs and account for many poisoning cases. Examples include aspirin and acetaminophen. Aspirin interferes with the oxidative burning of fuel by cells. To get energy, the cells switch to a less efficient way of burning fuel that does not use oxygen but generates a lot of heat. Increased perspiration develops to counteract a rise in body temperature, leading to dehydration and thirst. Aspirin also alters the pH in the body, affecting the central nervous system (Table 3 (Drugs and health care products)). The major toxicity of acetaminophen is liver damage.
Drugs and health care productsThe major toxicity from narcotic analgesics, like morphine, is depression of the central nervous system, especially the brain centre controlling respiration. The cause of death in morphine overdoses is usually respiratory failure. Nausea is caused by morphine's stimulation of the chemoreceptor trigger zone in the brain, and constipation is caused by morphine's depression of muscular activity in the intestine (Table 3 (Drugs and health care products)).
Tranquilizers and sleeping pills
Drugs and health care products Drugs and health care productsBenzodiazepines, such as diazepam, clonazepam, and chloridazepoxide, have a wide margin of safety when used at prescribed doses. Their major toxic effect is depression of the central nervous system, which results in muscular incoordination and slurred speech (Table 3 (Drugs and health care products)). For sleeping pills containing barbiturates (barbiturate), chloral hydrate, paraldehyde, and meprobamate, however, the margin of safety is much narrower, and the major toxicity is severe depression of the central nervous system, leading to respiratory and cardiovascular failure (Table 3 (Drugs and health care products)).
Antipsychotic drugs
Drugs and health care productsLike benzodiazepines, antipsychotic drugs such as chlorpromazine, perphenazine, and haloperidol have a relatively large therapeutic index, rarely causing fatalities. They occasionally may block the action of the parasympathetic and sympathetic nervous systems and thus produce such undesired effects as dry mouth and blurred vision from the former and a drop in blood pressure upon standing in the latter (Table 3 (Drugs and health care products)).
Cold medications
Drugs and health care products Drugs and health care productsNasal decongestants, antihistamines, and cough medicine, which are found in over-the-counter preparations for treating the symptoms of colds, have a low potential to produce toxicity. Nasal decongestants, such as ephedrine, mimic the action of epinephrine by stimulating the sympathetic nervous system, and consequently, an overdose of ephedrine produces symptoms related to stimulation of the sympathetic and central nervous systems (Table 3 (Drugs and health care products)). Depression of the central nervous system and parasympathetic blockade are two common toxicities of antihistamines such as diphenhydramine (Table 3 (Drugs and health care products)). Depression of the central nervous system is also the major toxicity of dextromethorphan and codeine, both used to suppress coughing.
Antiseptics (antiseptic)
Drugs and health care productsMost antiseptics (e.g., hydrogen peroxide, benzoyl peroxide, resorcinol, benzalkonium chloride, parabens, and cetylpyridinium chloride) produce gastrointestinal irritation if ingested (Table 3 (Drugs and health care products)). Benzoyl peroxide and parabens applied to the skin may be toxic. Among the most toxic antiseptics are hexachlorophene, benzalkonium, and cetylpyridinium chloride, any of which can cause injuries to internal organs. Systemic toxicity (double vision, drowsiness, tremor, seizures, and death) with hexachlorophene is more likely to occur in babies because the relatively thin stratum corneum of their skin is highly permeable.
Vitamins (vitamin) and iron pills
Drugs and health care productsDeficiencies as well as excesses of vitamins are harmful. Excessive vitamin A (retinol, or retinoic acid), known as hypervitaminosis A, can result in skin lesions, edema, and liver damage. Overconsumption by Alaskan natives of polar bear liver, a rich source of vitamin A, has produced acute toxicities, characterized by irresistible sleepiness and severe headaches. Chronic poisoning with vitamin A can cause neurological symptoms, including pain, anorexia, fatigue, and irritability (Table 3 (Drugs and health care products)).
Drugs and health care productsExcess vitamin C can lead to kidney stones. Apart from irritation of the skin and respiratory tract, the most severe toxicity of vitamin K excess is the increased destruction of red blood cells, which leads to anemia and the accumulation of bilirubin, one of the products of hemoglobin degradation (Table 3 (Drugs and health care products)). Excess bilirubin can result in brain damage in newborns, a condition known as kernicterus. Because the blood–brain barrier is not well developed in newborns, bilirubin enters and damages the brain. Due to the blood–brain barrier, kernicterus is not seen in adults.
iron, a metal that is necessary for normal health, can also cause poisoning. The toxicity of iron is a result of its corrosive action on the stomach and intestine when present in high concentrations. As a result, intestinal bleeding occurs, which can lead to the development of shock.
Antidepressants (antidepressant)
Drugs and health care productsAmong tricyclic antidepressants, amitriptyline and imipramine account for most of the fatal cases of poisoning. These drugs have a number of effects, including blockage of the parasympathetic system and damage to the central nervous system, the latter producing symptoms such as fatigue, weakness, lowered body temperature, seizures, and respiratory depression (Table 3 (Drugs and health care products)). Death is usually caused by damage to the heart. Lithium salts, used to treat manic depression, have a relatively low therapeutic index.
Drugs of abuse
Mind-altering drugs commonly abused include amphetamines, cocaine, phencyclidine, heroin, and methaqualone. These drugs are primarily toxic to the central nervous system; amphetamine and cocaine cause stimulation of the system (hallucinations and delirium), and heroin causes the depression of the system (depressed respiration and coma). In contrast, phencyclidine and methaqualone are biphasic, producing first depression (drowsiness) and then stimulation of the central nervous system (delirium and seizures). Amphetamines also affect the gastrointestinal tract (anorexia, nausea, vomiting, diarrhea) and stimulate the cardiovascular system (increased blood pressure and heart rate, palpitations, and abnormal heart rhythm). In addition to hallucinations and delirium, cocaine causes euphoria, sexual arousal, confusion, and sympathetic stimulation. Phencyclidine is also known to cause aggression and psychotic behaviour, while methaqualone produces excessive dreaming and amnesia.
Cardiovascular drugs
Drugs and health care products digitalis (e.g., digoxin) is a class of drugs used for congestive heart failure, with a very narrow margin of safety. Digitalis overdose usually begins with gastrointestinal symptoms, such as anorexia, nausea, and vomiting, followed by sensory symptoms, such as pain and visual disturbances (Table 3 (Drugs and health care products)). There are also effects on the central nervous system, characterized by delirium and hallucinations.
Drugs and health care productsThe major toxicities of beta blockers (beta-blocker) (e.g, propranolol and metoprolol) result from the blockage of sympathetic effects on the tracheobronchial tree (lung) and heart. Sympathetic stimulation relaxes smooth muscles in the tracheobronchial wall and makes the heart beat faster and more forcefully. Blockage produced by propranolol or metoprolol can cause bronchoconstriction and heart failure (Table 3 (Drugs and health care products)).
Antiasthmatics
Drugs for treating asthma, such as theophylline and aminophylline, are structurally similar to caffeine. Like caffeine, which is a stimulant, theophylline and aminophylline also stimulate the central nervous system. Therefore, excitement, delirium, rapid breathing, increased heart rate, and seizures occur with an overdose. With excessive stimulation of the heart, palpitations and irregular heart rhythm (arrhythmia) can result, leading to sudden death.
Poisons of biological origin
Biotoxins can be conveniently grouped into three major categories: (1) microbial toxins (toxin), poisons (poison) produced by bacteria, blue-green algae, dinoflagellates, golden-brown algae, etc., (2) phytotoxins, poisons (poison) produced by plants, and (3) zootoxins, poisons produced by animals. The geographic distribution of poisonous organisms varies greatly; poison-producing microorganisms tend to be ubiquitous in their distribution. Poisonous plants and animals are found in greatest abundance and varieties in warm-temperate and tropical regions. Relatively few toxic organisms of any kind are found in polar latitudes.
Knowledge of the evolutionary (evolution) significance and development of most biotoxins is largely speculative and poorly understood. In some instances they may have developed during the evolution of certain animal species as part of the food procurement mechanism (e.g., in snakes; cnidarians, jellyfishes, and their relatives; mollusks, octopuses, and others; and spiders). Biotoxins may also function as defensive mechanisms, as in some snakes, fishes, arthropods (e.g., insects, millipedes), and others. The defense may be quite complex—as in the protection of territorial rights for reproductive purposes—and inhibitory or antibiotic substances may be produced that result in the exclusion of competitive animal or plant species. Certain marine organisms and terrestrial plants may release into the water, air, or soil inhibitory substances that discourage the growth of other organisms; well-known examples include the production of antibiotic substances by microorganisms. Similar chemical-warfare mechanisms are used in battles for territorial rights among the inhabitants of a coral reef, a field, or a forest. Thus biotoxins play important roles in the regulation of natural populations. Of increasing interest has been the discovery that certain substances, which may be toxic to one group of organisms, may serve a vital function in the life processes of the source organism.
Importance to humans
Venom-producing animals and stinging and dermatogenic (i.e., skin-poisoning) plants capable of inflicting pain and sometimes death by means of parenteral contact (i.e., by bringing poisons into the body other than through the digestive tract) constitute environmental hazards. Biotoxic agents may produce their injurious effects by becoming involved in the food supply; ingestion of a poisonous microbial organism, plant, or marine animal or one of their toxic by-products may cause intoxication. An example is that of the shore fishes of many tropical islands; otherwise valuable food fishes are frequently contaminated by a poison called ciguatoxin. The poison, a potent neurotoxin (nerve poison), is accidentally ingested by the fishes in their food; such fish can no longer be used for either human or animal consumption.
Some of the effects produced by biotoxins (pharmacology) on humans are of an acute nature, and the injuries they cause are readily discernible. The effects of some of the mycotoxins (mycotoxin) (poisons produced by fungi) and poisons produced by plants, however, are long-term and chronic; they result in the development of cancerous growths and other chronic degenerative changes that are sometimes difficult to detect.
Microbial toxins
Microbial poisons are produced by the Monera (moneran) (bacteria and blue-green algae) and Protista (protist) (algae, protozoa, and others), and the Fungi. Various classifications have been proposed for the microbial poisons, but none is entirely satisfactory. The problems encountered when dealing with these organisms result from a lack of precise knowledge concerning their biological nature and their phylogenetic relationships; in addition, their poisons show great diversity and chemical complexity. The following outline, however, is useful in dealing with this subject.
Moneran toxins
The prefixes “exo-” and “endo-” are retained in classifying the bacterial toxins mainly for historical reasons rather than because they are found either outside or inside the bacterial cell. The main differences in these toxins lie in their chemical structure.
Poisonous proteins (protein) from bacteria are sometimes referred to as bacterial exotoxins (exotoxin). The exotoxins are generally produced by gram-positive organisms (i.e., bacteria that react in certain ways to the staining procedure known as Gram staining); at least two bacteria, Shigella dysenteriae and Vibrio cholerae, that produce exotoxins are gram-negative, however. The exotoxins usually do not contain any nonprotein substances, and most are antigenic (antigen); i.e., they stimulate the formation of antibodies. The exotoxins may appear in the culture medium in which the bacteria are growing during the declining phases of growth; in some cases they are released at the time of normal destruction of the cells after death (autolysis). The exotoxins are less stable to heat than are the endotoxins, and they may be detoxified by agents that do not affect endotoxins. They are more toxic than endotoxins, and each exotoxin exerts specific effects which are collectively known as pharmacological properties. Exotoxins are neutralized by homologous antibodies—i.e., the active agents in blood serum produced by a process involving the bacteria against which the serum is to be used.
Endotoxins (endotoxin) are antigens composed of complexes of proteins, polysaccharides (polysaccharide) (large molecules built up of numerous sugars), and lipids (lipid) (fats). The protein part determines the antigenicity, or quality of being reacted against as a foreign substance in a living organism. The polysaccharide part determines the immunological specificity, or limitations on the types of antibodies that can react with the endotoxin molecule and neutralize it (the immunological reaction). Some of the lipids possibly determine the toxicity. Endotoxins are derived from the bacterial cell wall and, when cells are grown in culture, are released only on autolysis. Endotoxins are not neutralized by homologous antibodies and are relatively stable to heat; all of them have the same pharmacological properties.
The Cyanobacteria, or blue-green algae, are among the most primitive and widely distributed of all organisms. They have extreme temperature tolerances. Some strains of a species are toxic; other strains of the same species are not. Water blooms of blue-green algae have been responsible for the death of fishes, waterfowl, cattle, horses, swine, and other animals. Blue-green algae have also been implicated as causes of human intoxications.
Mycotoxins (mycotoxin)
Fungi (fungus) are plantlike members of the kingdom Fungi (Mycota) that do not contain chlorophyll. A significant number are known to produce poisons of various types. Toxic fungi can be roughly divided into two main categories on the basis of their size: the smaller microfungi and the larger mushrooms. The toxic microfungi are members of one of two classes: Ascomycetes (Ascomycota), or the sac fungi, and the deuteromycetes, or the imperfect fungi (i.e., fungi in which no sexual reproductive stages are known). The large toxic mushrooms, or toadstools, are mostly members of the class Basidiomycetes (Basidiomycota), although some Ascomycetes, such as the poisonous false morel (Gyromitra esculenta), may attain a size as large as some of the mushrooms.
Representative toxic microfungiThe ability of certain fungi, such as ergot (Claviceps purpurea) and some mushrooms, to produce intoxication has long been known. During the 19th century it was recognized that molds are responsible for such diseases as yellow-rice toxicoses in Japan and alimentary toxic aleukia in Russia. The eruption of so-called turkey X disease in England in 1960 and the resulting discovery of the substance known as aflatoxin (see Table 4 (Representative toxic microfungi)) stimulated study of the subject of mycotoxicology. Because mycotoxins have now been recognized as potential cancer-producing agents (carcinogens) that can become involved in man's food supply, they have become important in the study of environmental carcinogenesis.
Representative poisonous mushroomsPoisonous mushrooms (mushroom), or toadstools as they are commonly called, are the widely distributed members of the class Basidiomycetes, although only a few are known to be poisonous when eaten (see Table 5 (Representative poisonous mushrooms)); some of the poisons, however, are deadly. Most deaths attributed to mushroom poisoning result from eating members of the genus Amanita. Wild mushrooms should be eaten only if they have been accurately identified by an experienced person; the safest procedure is to eat only cultivated species. The problem of toxicity in mushrooms is complex; no single rule or test method exists by which the toxicity of a mushroom can be determined. The most poisonous species closely resemble some of the most prized edible species; in addition, toxicity within a given wild species may vary from one set of ecological conditions or from one geographical locality to the next. Moreover, although some mushrooms that are poisonous when fresh are edible when cooked, dried, salted, or preserved in some other way, others remain poisonous in spite of all preparation procedures. It has also been observed that some people may become poisoned by eating mushrooms that apparently do not affect others. As with microfungi, the mushroom poisons vary in their chemical and biological properties from species to species.
Protistan poisons
The dinoflagellates (dinoflagellate), important producers of the primary food supply of the sea, are microscopic one-celled organisms that are dependent upon various inorganic nutrients in the water and upon radiant energy for photosynthesis, the process by which they produce their own food supplies. Although dinoflagellates inhabit both marine waters and freshwaters, most species are marine. Dinoflagellates are most often found in cool or temperate waters. During periods of planktonic blooms (times of high concentrations of microscopic organisms in the water) dinoflagellates multiply in large numbers. These planktonic blooms, sometimes referred to as red tide because they discolour the water, are often associated with weather disturbances that may bring about changes in water masses or upwellings. During periods of bloom large numbers of toxic dinoflagellates may be ingested by shellfish; the poisons accumulate in their digestive glands. Animals and humans may in turn be poisoned by eating poisoned shellfish. Certain species of dinoflagellates are capable of producing some of the most toxic substances known. The two species of dinoflagellates most commonly involved in human intoxications have been Gonyaulax catenella along the Pacific coast of North America and G. tamarensis along the eastern coast of North America. Intoxications from these organisms are known as paralytic shellfish poisoning. The symptoms, which begin with a tingling or burning sensation, then numbness of the lips, gums, tongue, and face, gradually spread. Gastrointestinal upset may be present. Other symptoms include weakness, joint aches, and muscular paralysis; death may result. There is no specific treatment or antidote. The poison, variously called paralytic shellfish poison, mussel poison, and saxitoxin, is a complex nonprotein nitrogen-containing compound. Paralytic shellfish poisoning is best avoided by following local public-health quarantine regulations.
Respiratory irritation may result from the inhalation of toxic products in the windblown spray from red-tide areas containing the toxic dinoflagellate Gymnodinium breve, which is found in the Gulf of Mexico and Florida; the nature of the poison is unknown. Deaths of large numbers of brackish-water pond fishes because of Prymnesium parvum have been reported in Israel; the poison is known as prymnesin.
Plant poisons (phytotoxins)
Representative poisonous plantsThe study of plant poisons is known as phytotoxicology. Most of the poisonous higher plants are angiosperms (angiosperm), or flowering plants, but only a small percentage are recognized as poisonous. Several systems have been devised for the classification of poisonous plants, none of which is completely satisfactory. Poisonous plants may be classified according to the chemical nature of their toxic constituents, their phylogenetic relationship, or their botanical characteristics. The following classification, which is based on their toxic effects, has been found to be useful: (1) plants that are poisonous to eat, (2) plants that are poisonous upon contact, (3) plants that produce photosensitization, and (4) plants that produce airborne allergies (see Table 6 (Representative poisonous plants)).
Plant poisons, or phytotoxins, comprise a vast range of biologically active chemical substances, such as alkaloids (alkaloid), polypeptides, amines, glycosides, oxalates, resins, toxalbumins, and a large group of miscellaneous compounds whose chemical structure has not yet been determined. Alkaloids, most of which are found in plants, are characterized by the presence of nitrogen and their ability to combine with acids to form salts. They are usually bitter in taste. It has been estimated that about 10 percent of the plant species contain some type of alkaloid. Only a few of the 5,000 alkaloids characterized thus far do not produce any biological activity; most cause a strong physiological reaction when administered to an animal. Amines are organic compounds containing nitrogen. A polypeptide is a string of three or more amino acids. A few polypeptides and amines are toxic to animals. Some glycosides (glycoside), which are compounds that yield one or more sugars and one or more other compounds—aglycones (nonsugars)—when hydrolyzed (chemically degraded by the introduction of water molecules between adjacent subunits), are extremely toxic to animals. Toxicity resides in the aglycone component or a part of it. Oxalates are salts of oxalic acid, which under natural conditions is not toxic but becomes so because of the oxalate ion. Resins (resin), a heterogeneous assemblage of complex compounds, differ widely in chemical properties but have certain similar physical properties. Some resins are physiologically very active, causing irritation to nervous and muscle tissue. Toxalbumins are highly toxic protein molecules that are produced by only a small number of plants. Ricin, a toxalbumin from the castor bean (castor-oil plant) (Ricinus communis), is one of the most toxic substances known.
Under certain ecological conditions plants may become poisonous as a result of the accumulation of toxic inorganic minerals such as copper, lead, cadmium, fluorine, manganese, nitrates, or selenium. Photosensitization, an unusual toxic reaction resulting from the ingestion of certain plants, may be of two types. The toxic substance may be obtained directly from the plant, which thereupon acts on the skin (primary photosensitivity), or the toxicity may result from liver damage caused by the metabolism of a toxic plant and failure of the breakdown products to be eliminated by the liver (hepatic photosensitivity). In either case the animal reacts by becoming restless; in addition, the skin reddens, and a severe sloughing of the skin develops. Death seldom occurs.
Representative poisonous plantsA large number of poisonous plants occur throughout the world; a few representative species and their poisons are listed in Table 6 (Representative poisonous plants).
Animal poisons (zootoxins)
Poisonous animals are widely distributed throughout the animal kingdom; the only major group that seems to be exempt is the birds.
Zootoxins can be divided into several categories: (1) oral poisons—those that are poisonous when eaten; (2) parenteral poisons, or venoms (venom)—those that are produced by a specialized poison gland and administered by means of a venom apparatus; and (3) crinotoxins—those that are produced by a specialized poison gland but are merely released into the environment, usually by means of a pore.
Representative animals poisonous when eaten Representative venomous animals that inflict a sting Representative crinotoxic animalsOral zootoxins (see Table 7 (Representative animals poisonous when eaten)) are generally thought to be small molecules; most venoms (Table 8 (Representative venomous animals that inflict a sting)) are believed to be large molecules, usually a protein or a substance in close association with one. Venoms, which are produced by specialized poison glands, are injected by means of a mechanical device that is able to penetrate the flesh of the victim. Little is known about the biological or chemical properties of most crinotoxins (Table 9 (Representative crinotoxic animals)). The term poisonous may be used in the generic sense to refer to all three categories of zootoxins.
Some of the most complex relationships in biotoxicology are found in the marine environment. Certain marine biotoxins, such as ciguatera fish poison, apparently originate in marine plants, are ingested by herbivores and then passed on to carnivores and eventually to humans. The extremely complex mechanism by which this is accomplished is not clear. With the buildup of toxic industrial chemical pollutants in the marine environment, the problems of toxicity in marine organisms are becoming increasingly more serious. There is evidence that under certain conditions chemical pollutants may trigger biotoxicity cycles in marine organisms. The outbreaks in Japan of Minamata disease were the result of such a cycle: microorganisms, algae, shellfishes, and fishes ingested or absorbed industrial wastes with highly toxic organic compounds containing mercury and were in turn consumed by humans, causing a number of deaths among the population.
Representative animals poisonous when eaten Representative venomous animals that inflict a sting Representative crinotoxic animalsThe relationships of representative poisonous animals and their position in the total framework of the animal kingdom can best be appreciated by categorizing them according to the group in which they belong. They are further grouped as to whether they are poisonous to eat, venomous, or crinotoxic in Tables 7 (Representative animals poisonous when eaten), 8 (Representative venomous animals that inflict a sting), and 9 (Representative crinotoxic animals).
Radiation
Radiation, radioactivity, and radioisotopes
Radiation is a flow of energy through space or matter. It takes the form of particles (e.g., alpha and beta particles) or electromagnetic waves (e.g., X rays, gamma rays, and visible and ultraviolet 【UV】 light). radiation can be classified as either ionizing or nonionizing depending on its ability to produce ions in the matter it interacts with. Ionizing radiation is more toxic than nonionizing radiation.
Radioactivity is the emission of radiation caused by the disintegration of unstable nuclei of radioisotopes. After disintegration, a radioisotope may become a radioisotope of another element, which will further disintegrate. The disintegration series continues until a stable isotope is formed.
Ionizing radiation
Ionizing radiation is radiation that produces ions in matter during interaction with atoms in the matter. The toxic effect of ionizing radiation is related to the ionization. It is believed that ionization of tissues, composed mainly of water, generates H2O+ and H2O− ions, which in turn form H and OH radicals. Because radicals are very reactive chemically, biological damage, such as attacks on DNA and proteins, results.
There are two classes of ionizing radiation: particulate and eletromagnetic (electromagnetic radiation). Alpha particles, beta particles, neutrons, and positrons are examples of particulate ionizing radiation. Gamma rays and X rays are electromagnetic ionizing radiation.
Among particulate ionizing radiation, alpha and beta particles are the forms most commonly encountered in the environment and are biologically the most significant. Composed of two neutrons and two protons and thus containing a 2+ charge, alpha particles (alpha particle) are the heaviest ionizing particles. Although they do not penetrate tissue very well, alpha particles turn many atoms in their short paths into ions, producing intense tissue ionization.
In contrast to alpha particles, beta particles (beta particle) are electrons of little mass carrying only one negative charge. They penetrate up to several millimetres in soft tissues. Their low mass and low charge mean that only moderate ionization is produced in tissues when beta particles collide with atoms in its path.
Gamma rays (gamma ray) and X rays (X-ray) are electromagnetic radiation of similar properties, with gamma rays having higher energy than X rays. Gamma rays usually accompany the formation of alpha or beta particles. Neither gamma rays nor X rays carry a charge, and neither have mass; consequently, they can penetrate tissues easily, creating moderate ionization along their paths.
Biological damage is related to the degree of tissue ionization produced by radiation. Thus, a physical dose of alpha particles does not produce the same amount of damage as that produced by the same dose of beta particles, gamma rays, or X rays.
Radiation sources
Radiation is either natural or man-made. Natural radiation includes cosmic radiation, terrestrial radiation, radioisotopes inside human bodies, and radon gas. Cosmic radiation consists of charged particles from outer space, and terrestrial radiation of gamma rays from radionuclides in the Earth. Radioisotopes (radioactive isotope) in human bodies come from the food, water, and air consumed. Cosmic and terrestrial radiation, together with radioisotopes inside human bodies, contribute only one-third of the total natural radiation dose. The remaining two-thirds can be attributed to radon, a radioactive gas released from soil that may reach a high level inside buildings with poor ventilation. Man-made radiation consists of radiation from medical and dental diagnostic procedures, atmospheric tests of atomic bombs, emissions from nuclear plants, certain occupational activities, and some consumer products. The largest nonoccupational radiation sources are tobacco smoke for smokers and indoor radon gas for the nonsmoking population.
Emissions from nuclear (nuclear reactor) power plants contribute only a very small portion of the total yearly radiation received. The low dose reflects the negligible amount of radionuclides released during normal operation, although the amount released can be much higher after a nuclear reactor accident. Not every reactor accident is a disaster, however. The 1979 accident at the Three Mile Island nuclear power station, near Harrisburg, Pa., released only a small amount of radiation (0.8 and 0.015 mSv within a 16- and 80-km radius, respectively), less than the background annual radiation dose. The nuclear reactor accident at Chernobyl (Chernobyl accident) in the Soviet Union, in 1986, however, was much more devastating, leading to more than 30 deaths and the evacuation of thousands of nearby residents.
Adverse effects of ionizing radiation
Ionizing radiation quickly kills rapidly dividing cells. In general, immature blood cells in bone marrow, cells lining the mucosa of the gastrointestinal tract, and cells in the lower layers of the epidermis and in hair follicles are the most rapidly dividing cells in the body. As a result, radiation leads to the decreased production of blood cells, nausea, vomiting, diarrhea, malabsorption by the intestine, skin burns, and hair loss. Because of its relatively selective lethal effect on rapidly dividing cells, however, ionizing radiation is used in the treatment of certain cancers. Some cells in the embryo and fetus also divide rapidly, and thus ionizing radiation can cause malformations and even fetal death. Ionizing radiation can also produce mutations by altering the DNA, and it can result in cancer.
Toxicities of whole-body ionizing radiation
X rays and gamma rays irradiate the body uniformly and acutely affect all of the tissues discussed above. At sufficiently high doses, this type of radiation can lead to a condition known as acute radiation syndrome (ionizing radiation injury). The most sensitive tissue is the bone marrow, where blood cells are generated. The next tissue affected is the gastrointestinal tract. If the dose is high, the central nervous system is affected and the person becomes uncoordinated and disoriented and experiences tremors, convulsions, and coma. At even higher doses, the skin, eyes, and ovaries and testes are affected. Death may follow from 2 to 35 days after exposure. Exposure to radiation can also result in cancers of the bone marrow (leading to leukemia), lungs, kidneys, bladder, esophagus, stomach, colon, thyroid, or breasts.
Radioisotopes (radioactive isotope) that are absorbed and distributed evenly throughout the body also can result in whole-body irradiation. Examples are tritium and cesium-137, both of which release beta particles that can lead to bone marrow toxicities and even, in the case of cesium-137, to death. The toxicity of tritium is less severe than that of cesium-137 because the beta particles generated by tritium are less energetic and because cesium-137 also releases gamma rays.
Local toxicities of common beta-particle emitters
Unlike tritium and cesium-137, the isotopes strontium-90, iodine-131, and cerium-144 emit beta particles (beta particle) that are not distributed evenly in the body. Strontium-90 releases only beta particles, while iodine-131 and cerium-144 release both beta particles and gamma rays, but their toxicities are primarily caused by the beta particles. These radioisotopes produce toxicities in the tissues where they are stored or concentrated. Strontium-90 and cerium-144 chemically resemble calcium and as a result are stored in bone. Therefore, these two radioisotopes produce bone cancer and leukemia, which is a result of the irradiation of bone marrow. Iodine-131 is concentrated in the thyroid and produces thyroid damage and tumours.
Local toxicities of common alpha-particle emitters
There are radioisotopes that emit primarily alpha particles (alpha particle), together with some gamma rays. Because the destructive effect on tissues of alpha particles is far greater than that of gamma rays, the toxicities of these radioisotopes are contributed mainly by the alpha particles. Because of the limited penetrability of alpha particles, only tissues in the near vicinity of the isotopic molecules are affected. These radioisotopes typically produce tumours at the storage site.
Most of the common alpha-particle emitters belong to the uranium series, which consists of radioisotopes that form one after another, via a nuclear decay reaction, and release mainly alpha particles. The series starts with uranium-238. The nuclear disintegration of uranium-238 forms radium-226 which disintegrates to form radon gas (radon-222). Radon decays to form a series of daughter nuclides, most of which are alpha-particle-releasing isotopes, such as polonium-210. The radioisotopes in the uranium series are important because uranium is the starting fuel for many nuclear reactors and because daughter nuclides in this series are commonly found in the environment.
The toxicity of uranium-238 depends on the water solubility of the uranium compound. Water-soluble forms mainly cause kidney injury, while the insoluble forms produce fibrosis and cancer of the lung. Because of its similarity to calcium, radium-226 is stored mainly in the bone, and it produces abnormal changes in the bone marrow, including anemia and leukemia, cancers of the bone, and paranasal sinuses. The next radioisotope in the uranium series is radon, radon-222. Although radon is radioactive, its toxicity is not due to retention of the gas by the lungs but rather to the series of radioactive daughter nuclides in particulate form. These particulate daughter nuclides are deposited on the respiratory tract when inhaled, the respiratory tract is irradiated by the alpha particles released, and lung cancer can result.
Other radioisotopes do not belong to the uranium series. For example, radium-224, which is deposited mainly on bone surfaces, has been used in Europe to treat ankylosing spondylitis. Because of its short half-life (3.6 days), it affects only the bone surface and not the bone marrow. Its major toxicity is the production of bone cancer. Like uranium-238, plutonium-239, which is used in some nuclear reactors and in nuclear bombs, primarily releases alpha particles. Although there are no human data, animal studies indicate that the toxicity of plutonium-239 is similar to that of insoluble uranium-238 in causing fibrosis and cancer of the lung.
Nonionizing radiation
Nonionizing radiation includes ultraviolet light, infrared radiation, microwaves, and radio frequencies, all of which are electromagnetic waves. The toxicity of radio frequencies is rather low. On the whole, nonionizing radiation is not as toxic as ionizing radiation, and the various forms of nonionization radiation share common target organs; particularly the skin and eyes.
ultraviolet radiation
The toxicity of ultraviolet light depends on its wavelength. Ultraviolet-A (near UV) has a wavelength of 315–400 nanometres, ultraviolet-B (mid UV) has one of 280–315 nanometres, and ultraviolet-C (far UV) has one of 200–280 nanometres. Ultraviolet-A affects primarily the skin and causes burns at high energy levels. The toxicities of ultraviolet-B and ultraviolet-C are similar, but ultraviolet-C is less toxic because it does not penetrate tissues as deeply. Both ultraviolet-B and ultraviolet-C cause injuries to the eyes and skin. Ultraviolet-B is the major component of sunlight and accelerates the aging of skin by damaging the collagen fibres under it. Ultraviolet-B also is the cause of an occupational disease known as “welder's flash,” or “arc eye,” which is characterized by photophobia, tears in the eyes, spasm of the eyelids, and eye inflammation. Finally, ultraviolet-B can cause skin cancer, which may be a result of the linking of thymidines, a base in DNA, produced by ultraviolet-B radiation.
infrared radiation and microwaves
The major mechanism of toxicity of infrared radiation and microwaves is the production of heat in tissues. Infrared-A (wavelength 0.8–1.4 micrometres) penetrates the skin, causing burns and pigmentation. Infrared-A also penetrates the liquid content of the eye to reach the retina and can therefore produce damage to all parts of the eye. In contrast, infrared-B and infrared-C (wavelength 1.4–3,000 micrometres) are almost completely absorbed by the superficial layers of the skin and eyes, and the damage is thus confined to the surface. Microwaves (wavelength 1 millimetre to 1 metre) produce heat in tissues. Because testes and eyes do not dissipate heat well, due to low blood flow through these organs, temporary sterility and cataracts can be produced by microwaves.
Lasers (laser)
Lasers are high-energy light beams, visible and nonvisible, generated by atoms at an excited state and further amplified by optics. Like most other nonionizing radiation, lasers can produce skin burns. Visible lasers, with a wavelength from 0.4 to 1.4 micrometres, will cause retinal damage if they enter the eyes and are focused by the lens onto the retina.
Additional Reading
Toxic substances
Curtis D. Klaassen, Mary O. Amdur, and John Doull (eds.), Casarett and Doull's Toxicology: The Basic Science of Poisons, 3rd ed. (1986), contains in-depth discussions of basic toxicology principles and information on toxins classified according to use and target organs. Analysis of the chemical structure of toxins influencing their effect is provided in Stanley E. Manahan, Toxicological Chemistry: A Guide to Toxic Substances in Chemistry (1989). Michael A. Kamrin, Toxicology: A Primer on Toxicology Principles and Applications (1988), is a concise, nontechnical general introduction. Ernest Hodgson, Richard B. Mailman, and Janice E. Chambers, Dictionary of Toxicology (1988), explains concepts and terminology and covers organizations and authorities in the field.
Exposure and response to poisons
Robert H. Dreisbach and William O. Robertson, Handbook of Poisoning: Prevention, Diagnosis, & Treatment, 12th ed. (1987), contains concise but essential information on the toxicity and treatment of poisoning by biological toxins and drugs. Avram Goldstein, Lewis Aronow, and Sumner M. Kalman, Principles of Drug Action: The Basis of Pharmacology, 2nd ed. (1973), describes the principles governing chemical absorption, distribution, and excretion of the substances. Matthew J. Ellenhorn and Donald G. Barceloux, Medical Toxicology: Diagnosis and Treatments of Human Poisoning (1988), focuses on the poisons derived from biological sources. Sidney Kaye, Handbook of Emergency Toxicology: A Guide for the Identification, Diagnosis, and Treatment of Poisoning, 5th ed. (1988), surveys almost 200 toxic substances.
Sources of poisoning
George D. Clayton and Florence E. Clayton (eds.), Patty's Industrial Hygiene and Toxicology, 3rd. rev. ed., vol. 2, Toxicology, parts A, B, and C (1981–82), is an extensive compendium of information on the toxicology of industrial chemicals. For concise reference on the subject, see Alice Hamilton, Hamilton and Hardy's Industrial Toxicology, 4th ed., rev. by Asher J. Finkel (1983). The toxicology of chemicals found in commercial products is examined in Robert E. Gosselin, Roger P. Smith, and Harold C. Hodge, Clinical Toxicology of Commercial Products, 5th ed. (1984), where information on chemical ingredients of many commercial products is detailed. Goodman & Gilman's The Pharmacological Basis of Therapeutics, 9th ed. by Joel G. Hardman and Lee E. Limbird (1996), is an authoritative source of information on the toxicology of drugs and includes a section on the toxicity of industrial chemicals.The toxicology of food is the subject of Joint FAO/WHO Committee on Food Additives, Toxicological Evaluation of Certain Food Additives (1988), including lists of acceptable consumption of flavourings, preservatives, and colours; Jose M. Concon, Food Toxicology, 2 vol. (1988), a comprehensive survey of food contamination and poisoning; Palle Krogh (ed.), Mycotoxins in Food (1987); and W.F.O. Marasas and Paul E. Nelson, Mycotoxicology: Introduction to the Mycology, Plant Pathology, Chemistry, Toxicology, and Pathology of Naturally Occuring Mycotoxicoses in Animals and Man (1987). Bruce W. Halstead, Poisonous and Venomous Marine Animals of the World, 2nd rev. ed. (1988), is an exhaustive and profusely illustrated compendium on the toxic marine animals of the world, from protozoans to polar bears, including their historical background, names, geographic distribution, biology, mechanism of intoxication, and medical, toxicological, pharmacological, and chemical aspects.