thorium processing
Introduction
preparation of the ore for use in various products.
Thorium (Th) is a dense (11.7 grams per cubic centimetre), silvery metal that is softer than steel. It has a high melting temperature of approximately 1,750° C (3,180° F). Below about 1,360° C (2,480° F), the metal exists in the face-centred cubic (fcc) crystalline form; at higher temperatures up to its melting point, it takes on the body-centred cubic (bcc) form. Finely divided thorium metal will burn in air, but the massive metal is stable in air at ordinary temperatures (although it will react with oxygen to form a surface tarnish after prolonged exposure). Because of its reactivity, it is extracted from minerals only with difficulty.
Almost all thorium found in nature is the isotope thorium-232 (several other isotopes exist in trace amounts or can be produced synthetically). This slightly radioactive material is not fissile itself, but it can be transformed in a nuclear reactor to the fissile uranium-233. Since thorium is present in the Earth's crust in about three times the quantity of uranium, its fertile quality represents a virtually unlimited source of nuclear energy. In order for this theoretical value to be realized, however, the barriers of costly extraction and conversion techniques would have to be overcome.
History
The Swedish chemist Jöns Jacob Berzelius discovered thorium in 1828, successfully isolating the metal from the silicate mineral now known as thorite. The name thorium originates from Thor, the Germanic war god. After the development of the incandescent gas mantle by Carl Auer, Baron von Welsbach, in the 1880s, thorium came into extensive demand and use, but, when electric power became generally available after 1920, worldwide utilization of thorium gas mantles sharply declined.
In the early 1950s Spedding and his associates at the Ames (Iowa) Laboratory of the U.S. Atomic Energy Commission developed a practicable and efficent method for large-scale preparation of thorium metal. The Spedding procedure involves reduction of mixtures of zinc halides and thorium tetrafluoride with calcium metal.
Ores
The major commercial source of thorium is monazite, an anhydrous rare-earth phosphate with the chemical formula (Ce,La,Nd,Th)PO4. Typically, 3 to 5 percent of the metal content of monazite is thorium (in the form of thorium dioxide, ThO2). Much of the world's current demand for thorium metal and its compounds is satisfied by mining placers along India's Malabar Coast, where wave action deposits monazite as a coarse yellow-to-brown sand on beaches. Other ores of thorium are the oxide mineral thorianite (ThO2) and the silicate mineral thorite (ThSiO4); these are not commercially mined.
Mining and concentrating
Monazite beach sands are readily mined with conventional placer mining equipment and procedures. The dredged monazite is admixed with a variety of other minerals, including silica, magnetitie, ilmenite, zircon, and garnet. Concentration is accomplished by washing out lighter minerals in shaking tables and passing the resulting monazite fraction through a series of electromagnetic separators, which separate monazite from other minerals by virtue of their different magnetic permeabilities.
Extraction and refining
Acidic and alkaline digestion
Although monazite is very stable chemically, it is susceptible to attack by both strong mineral acids (acid) (e.g., sulfuric acid, H2SO4) and alkalies (e.g., sodium hydroxide, NaOH). In the acid treatment, finely ground monazite sand is digested at 155° to 230° C (310° to 445° F) with highly concentrated (93 percent) H2SO4. This converts both the phosphate and the metal content of the monazite to water-soluble species. The resulting solution is contacted with aqueous ammonia, first precipitating hydrated thorium phosphate as a gelatinous mass and then metathesizing the thorium phosphate to thorium hydroxide. Finally, the crude thorium hydroxide is dissolved in nitric acid to produce a thorium nitrate-containing feed solution suitable for final purification by solvent extraction (see below).
In alkaline (alkali) digestion, finely ground monazite sand is carefully treated with a concentrated NaOH solution at 138° C (280° F) to produce a solid hydroxide product. Any one of several mineral acids is then used to dissolve this solid residue. For example, treatment with hydrochloric acid yields a solution of thorium and rare-earth chlorides. Conventionally, thorium is partially separated from the rare earths by addition of NaOH to the acidic chloride solution. The crude thorium hydroxide precipitate is then dissolved in nitric acid for final purification by solvent extraction.
Solvent extraction
For the purification of thorium from residual rare earths and other contaminants present in nitric acid feed solutions, the crude thorium nitrate concentrate is usually contacted with a solution of tributyl phosphate diluted by a suitable hydrocarbon. The resulting organic extract, containing the thorium (and any uranium that may be present), is then contacted countercurrently with a small volume of nitric acid solution in order to remove contaminating rare earths and other metallic impurities to acceptable levels. Finally, the scrubbed tributyl phosphate solution is contacted with a dilute nitric acid solution; this removes, or strips, thorium from the organic solvent into the aqueous solution while retaining uranium (if present) in the organic phase. Thermal concentration of the purified thorium nitrate solution yields a product suitable for the fabrication of gas mantles (see below Chemical compounds). The nitrate can also be calcined to ThO2, which is incorporated into ceramic fuel elements for nuclear reactors or is converted to thorium metal.
Reduction to the metal
Powdered ThO2 can be fluorinated with gaseous hydrogen fluoride (HF), yielding thorium tetrafluoride (ThF4). The metal is obtained by the Spedding process, in which powdered ThF4 is mixed with finely divided calcium (Ca) and a zinc halide (either zinc chloride or zinc fluoride) and placed in a sealed, refractory-lined “bomb.” Upon heating to approximately 650° C (1,200° F), an exothermic reaction ensues that reduces the thorium and zinc to metal and produces a slag of calcium chloride or calcium fluoride:
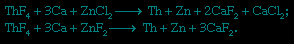
After solidification, the zinc-thorium alloy product is heated above the boiling point of zinc (907° C, or 1,665° F) but below the melting temperature of thorium. This evaporates the zinc and leaves a highly purified thorium sponge, which is melted and cast into ingots.
Conversion to uranium-233
When bombarded by thermalized neutrons (usually released by the fission of uranium-235 in a nuclear reactor), thorium-232 is converted to thorium-233. This isotope decays to protactinium-233, which in turn decays to uranium-233:
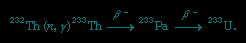
The fissile properties of uranium-233 can be utilized immediately or after recovery from the irradiated reactor fuel.
Uranium-233 can be recovered and purified from neutron-irradiated thorium reactor fuels through the thorium extraction, or Thorex, process, which employs tributyl phosphate extraction chemistry. Irradiated fuel, containing either thorium metal or oxide, is dissolved in nitric acid containing a small amount of fluoride ion. Uranium-233 and thorium are coextracted into a tributyl phosphate solution, which is then contacted with an aluminum nitrate solution to remove traces of accompanying fission products. Dilute nitric acid is used to preferentially remove thorium from the scrubbed organic phase. Uranium-233 remaining in the tributyl phosphate solvent is stripped into acidified water; the resulting strip solution is passed through an ion-exchange resin bed in order to concentrate and purify the uranium-233.
The metal and its alloys (alloy)
Thorium is reported to alloy readily with many elements, including aluminum, beryllium, bismuth, boron, cobalt, copper, gold, iron, lead, magnesium, mercury, molybdenum, nickel, platinum, selenium, silver, sodium, tantalum, tungsten, and zinc. Some thorium is alloyed with magnesium metal to produce a material of increased high-temperature strength.
Chemical compounds
Thorium nitrate
Aqueous solutions of highly purified thorium nitrate, Th(NO3)4, are produced when thorium ores are processed (see above Extraction and refining). The nitrate is extensively used in the commercial production of gas mantles. Such mantles are made by impregnating cotton or synthetic fibres with a 25 to 50 percent solution of Th(NO3)4 containing 0.5 to 1 percent each of thorium sulfate and cerous nitrate. The impregnated fibres are treated with aqueous ammonia, producing thorium hydroxide, Th(OH)4, and this compound is calcined to produce ThO2. The latter substance, when heated, emits brilliant white light. The added cerous nitrate improves spectral emission properties, while the small amounts of thorium sulfate yield mantles with improved mechanical properties.
Thorium dioxide
The only other thorium compound of any industrial significance is ThO2, known as thoria. For nuclear applications, thoria is prepared by calcination of thoroughly purified Th(NO3)4.
Thoria also finds some application as a refractory material in various high-temperature processes.
Additional Reading
Comprehensive and up-to-date information on many aspects of metallurgy, individual metals, and alloys can be found in convenient reference-form arrangement in the following works: Metals Handbook, 9th ed., 17 vol. (1978–89), a massive and detailed source prepared under the direction of the American Society for Metals, with a 10th edition that began publication in 1990; Herman F. Mark et al. (eds.), Encyclopedia of Chemical Technology, 3rd ed., 31 vol. (1978–84), formerly known as Kirk-Othmer Encyclopedia of Chemical Technology, with a 4th edition begun in 1991; and its European counterpart, the first English-language edition of a monumental German work, Ullmann's Encyclopedia of Industrial Chemistry, 5th, completely rev. ed., edited by Wolfgang Gerhartz et al. (1985– ). Ed. Joseph J. Katz, Glenn T. Seaborg, and Lester L. Morss, The Chemistry of the Actinide Elements, 2nd ed., 2 vol. (1986), presents both the theoretical and descriptive features of the chemistry of thorium, uranium, and plutonium in a logical and extremely well-written manner; the coverage is extensive—from properties of individual isotopes to the technology for reprocessing irradiated reactor fuels—and is very lucid. Gmelins Handbuch der anorganischen Chemie, 8th ed., vol. A1 (1979), A3 (1981), and A4 (1982), is unquestionably the most comprehensive and authoritative source of information and data for all facets of thorium, uranium, and plutonium chemistry, physics, and metallurgy; compiled by recognized world-class experts and updated regularly, these volumes are the definitive reference in the field.
- New Tōkaidō Line
- New Ulm
- New Wave
- new wave
- New Westminster
- New Windsor
- New Year festival
- New York
- New York Botanical Garden
- New York Central Railroad Company
- New York, Chicago and St. Louis Railroad Company
- New York City
- New York City Ballet
- New York Daily News
- New York Dolls, the
- New Yorker, The
- New York, flag of
- New York Giants
- New York Herald
- New-York Historical Society
- New York Jets
- New York Mets
- New York, New Haven and Hartford Railroad Company
- New York Philharmonic
- New York Public Library