francium
chemical element
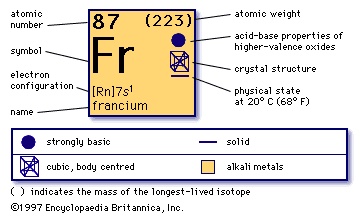 heaviest chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. It exists only in short-lived radioactive forms. Natural francium cannot be isolated in visible, weighable amounts, for only 24.5 grams (0.86 ounce) occur at any time in the entire crust of Earth. The existence of francium was predicted by Russian chemist Dmitry I. Mendeleyev in his periodic classification of the elements. French chemist Marguerite Perey discovered francium (1939) while studying actinium-227, which decays by negative beta decay ( electron emission) to an isotope of thorium (thorium-227) and by alpha emission (about 1 percent) into an isotope of francium (francium-223) that was formerly called actinium K (AcK) and is a member of the actinium decay series. Though it is the longest-lived isotope of francium, francium-223 has a half-life of only 22 minutes. Nineteen isotopes of francium with masses between 204 and 224 have been artificially prepared, and, because natural francium cannot be concentrated, it is also prepared by neutron irradiation of radium to produce actinium, which decays to produce traces of francium. The chemistry of francium can be studied only by methods designed for trace quantities. In all respects, its observed behaviour, including the oxidation state of +1, is that to be expected of an alkali element filling a place just below cesium in the periodic table of the elements. There is almost no information on its biological aspects.
heaviest chemical element of Group 1 (Ia) in the periodic table, the alkali metal group. It exists only in short-lived radioactive forms. Natural francium cannot be isolated in visible, weighable amounts, for only 24.5 grams (0.86 ounce) occur at any time in the entire crust of Earth. The existence of francium was predicted by Russian chemist Dmitry I. Mendeleyev in his periodic classification of the elements. French chemist Marguerite Perey discovered francium (1939) while studying actinium-227, which decays by negative beta decay ( electron emission) to an isotope of thorium (thorium-227) and by alpha emission (about 1 percent) into an isotope of francium (francium-223) that was formerly called actinium K (AcK) and is a member of the actinium decay series. Though it is the longest-lived isotope of francium, francium-223 has a half-life of only 22 minutes. Nineteen isotopes of francium with masses between 204 and 224 have been artificially prepared, and, because natural francium cannot be concentrated, it is also prepared by neutron irradiation of radium to produce actinium, which decays to produce traces of francium. The chemistry of francium can be studied only by methods designed for trace quantities. In all respects, its observed behaviour, including the oxidation state of +1, is that to be expected of an alkali element filling a place just below cesium in the periodic table of the elements. There is almost no information on its biological aspects.atomic number
87
stablest isotope
(223)
oxidation state
+1
electronic config.
2-8-18-32-18-8-1 or 【Rn】 7s1
- Porfirio Díaz
- porgy
- Pori
- Porirua
- Po River
- pork
- porkpie
- pornography
- porphyria
- porphyrin
- Porphyry
- porphyry copper deposit
- porpoise
- Porpora, Nicola
- Porres, Saint Martín de
- porringer
- Porsangen
- Porsche, Ferdinand
- Porsgrunn
- Porson, Richard
- port
- Port Adelaide Enfield
- Portage
- Porta, Giacomo della
- Porta, Giambattista della