gadolinium
chemical element
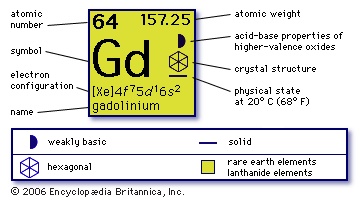 (Gd), chemical element, rare-earth metal of the lanthanoid series of the periodic table. Silvery white and moderately ductile, the metal reacts slowly with oxygen and water. Below 17° C it is ferromagnetic and at very low temperatures, superconducting. Credit for the discovery of gadolinium is shared by J.-C.-G. de Marignac (Marignac, Jean-Charles-Galinard de) and P.-É. Lecoq de Boisbaudran (Lecoq de Boisbaudran, Paul-Émile). Marignac separated (1880) a new rare earth (metallic oxide) from the mineral samarskite; Lecoq de Boisbaudran obtained (1886) a fairly pure sample of the same earth, which with Marignac's assent he named gadolinia, after a mineral in which it occurs that in turn had been named for the Finnish chemist Johan Gadolin. Gadolinium occurs in many minerals along with the other rare earths but is obtained primarily from monazite. It is found also in the products of nuclear fission. Commercial separation depends upon ion-exchange techniques. The metal has been produced by thermoreduction of the anhydrous chloride or fluoride by calcium.
(Gd), chemical element, rare-earth metal of the lanthanoid series of the periodic table. Silvery white and moderately ductile, the metal reacts slowly with oxygen and water. Below 17° C it is ferromagnetic and at very low temperatures, superconducting. Credit for the discovery of gadolinium is shared by J.-C.-G. de Marignac (Marignac, Jean-Charles-Galinard de) and P.-É. Lecoq de Boisbaudran (Lecoq de Boisbaudran, Paul-Émile). Marignac separated (1880) a new rare earth (metallic oxide) from the mineral samarskite; Lecoq de Boisbaudran obtained (1886) a fairly pure sample of the same earth, which with Marignac's assent he named gadolinia, after a mineral in which it occurs that in turn had been named for the Finnish chemist Johan Gadolin. Gadolinium occurs in many minerals along with the other rare earths but is obtained primarily from monazite. It is found also in the products of nuclear fission. Commercial separation depends upon ion-exchange techniques. The metal has been produced by thermoreduction of the anhydrous chloride or fluoride by calcium.Gadolinium is used for certain electronic components and high-temperature refractories and as an alloying agent. H. Kamerlingh Onnes (Kamerlingh Onnes, Heike) first produced (1923) temperatures below 1 K by magnetic cooling using gadolinium sulfate. Gadolinium has the highest absorption cross section for thermal neutrons of any natural isotope of any element (49,000 barns), which suggests its use in nuclear reactor control rods. The seven natural stable isotopes have mass numbers between 152 and 160; the species of highest mass numbers are more abundant.
Gadolinium displays the oxidation state +3 in all of its compounds; it behaves as a typical rare earth. Its salts are white, and its solutions are colourless.
atomic number
64
atomic weight
157.250
melting point
1,311° C
boiling point
3,233° C
specific gravity
7.898 (25° C)
oxidation state
+3
electronic config.
【Xe】4f 75d16s2
- Bosch, Hiëronymus
- Bosch, Johannes, graaf van den
- Bosch, Juan
- Bosch, Robert
- Bosco, Saint John
- Boscovich, Ruggero Giuseppe
- Boscán, Juan
- Bose-Einstein condensate
- Bose-Einstein statistics
- Boselli, Paolo
- Bose, Satyendra Nath
- Bose, Sir Jagadis Chandra
- Bose, Subhas Chandra
- boshan xianglu
- Bosman, Herman Charles
- Bosna River
- Bosnia and Herzegovina
- Bosnia and Herzegovina, flag of
- Bosnian crisis of 1908
- Boso
- boson
- Bosporus
- Bosporus, Kingdom of the
- boss
- bossa nova