greenhouse gas
atmospheric science
Introduction
 any gas that has the property of absorbing infrared radiation (net heat energy (heat)) emitted from Earth's surface and reradiating it back to Earth's surface, thus contributing to the phenomenon known as the greenhouse effect. carbon dioxide, methane, and water vapour are the most important greenhouse gases. To a lesser extent, surface-level ozone, nitrous oxides (nitrous oxide), and fluorinated gases also trap infrared radiation. Greenhouse gases have a profound effect on the energy budget of the Earth system despite making up only a fraction of all atmospheric gases. Concentrations of greenhouse gases have varied substantially during Earth's history, and these variations have driven substantial climate changes (climate change) at a wide range of timescales. In general, greenhouse gas concentrations have been particularly high during warm periods and low during cold phases.
any gas that has the property of absorbing infrared radiation (net heat energy (heat)) emitted from Earth's surface and reradiating it back to Earth's surface, thus contributing to the phenomenon known as the greenhouse effect. carbon dioxide, methane, and water vapour are the most important greenhouse gases. To a lesser extent, surface-level ozone, nitrous oxides (nitrous oxide), and fluorinated gases also trap infrared radiation. Greenhouse gases have a profound effect on the energy budget of the Earth system despite making up only a fraction of all atmospheric gases. Concentrations of greenhouse gases have varied substantially during Earth's history, and these variations have driven substantial climate changes (climate change) at a wide range of timescales. In general, greenhouse gas concentrations have been particularly high during warm periods and low during cold phases.A number of processes influence greenhouse gas concentrations. Some, such as tectonic activities (tectonics), operate at timescales of millions of years, whereas others, such as vegetation, soil, wetland, and ocean sources and sinks, operate at timescales of hundreds to thousands of years. Human activities—especially fossil-fuel (fossil fuel) combustion since the Industrial Revolution—are responsible for steady increases in atmospheric concentrations of various greenhouse gases, especially carbon dioxide, methane, ozone, and chlorofluorocarbons (chlorofluorocarbon) (CFCs).
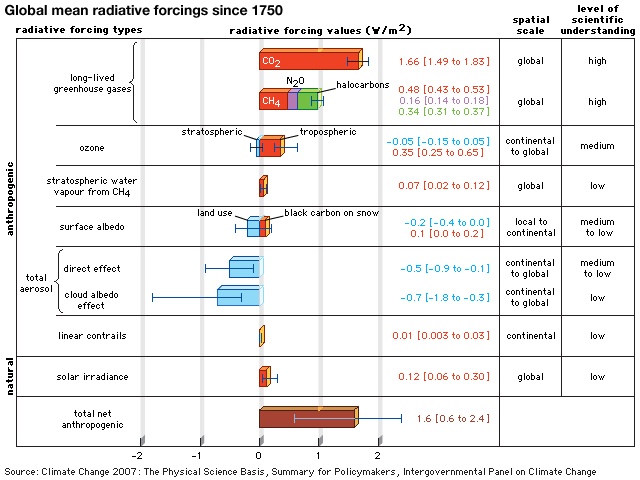
The effect of each greenhouse gas on Earth's climate depends on its chemical nature and its relative concentration in Earth's atmosphere. Some gases have a high capacity for absorbing infrared radiation or occur in significant quantities, whereas others have considerably lower capacities for absorption or occur only in trace amounts. Radiative forcing, as defined by the Intergovernmental Panel on Climate Change (IPCC), is a measure of the influence a given greenhouse gas or other climatic factor (such as solar irradiance or albedo) has on the amount of downward-directed radiant energy impinging upon Earth's surface. To understand the relative influence of each greenhouse gas, so-called forcing values (which are displayed in watts (watt) per square metre) calculated for the time period between 1750 and the present day are given below.
Major greenhouse gases
Water vapour
 water vapour is the most potent of the greenhouse gases in Earth's atmosphere, but its behaviour is fundamentally different from that of the other greenhouse gases. The primary role of water vapour is not as a direct agent of radiative forcing (global warming) but rather as a climate feedback—that is, as a response within the climate system that influences the system's continued activity. This distinction arises from the fact that the amount of water vapour in the atmosphere cannot, in general, be directly modified by human behaviour but is instead set by air temperatures. The warmer the surface, the greater the evaporation rate of water from the surface. As a result, increased evaporation leads to a greater concentration of water vapour in the lower atmosphere capable of absorbing infrared radiation and emitting it downward.
water vapour is the most potent of the greenhouse gases in Earth's atmosphere, but its behaviour is fundamentally different from that of the other greenhouse gases. The primary role of water vapour is not as a direct agent of radiative forcing (global warming) but rather as a climate feedback—that is, as a response within the climate system that influences the system's continued activity. This distinction arises from the fact that the amount of water vapour in the atmosphere cannot, in general, be directly modified by human behaviour but is instead set by air temperatures. The warmer the surface, the greater the evaporation rate of water from the surface. As a result, increased evaporation leads to a greater concentration of water vapour in the lower atmosphere capable of absorbing infrared radiation and emitting it downward.Carbon dioxide

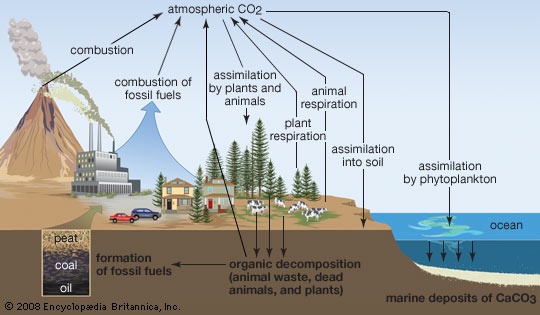 Of the greenhouse gases, carbon dioxide (CO2) is most significant. Natural sources of atmospheric CO2 include outgassing from volcanoes (volcano), the combustion and natural decay of organic matter, and respiration by aerobic (oxygen-using) organisms. These sources are balanced, on average, by a set of physical, chemical, or biological processes, called “sinks,” that tend to remove CO2 from the atmosphere. Significant natural sinks include terrestrial vegetation, which takes up CO2 during the process of photosynthesis.
Of the greenhouse gases, carbon dioxide (CO2) is most significant. Natural sources of atmospheric CO2 include outgassing from volcanoes (volcano), the combustion and natural decay of organic matter, and respiration by aerobic (oxygen-using) organisms. These sources are balanced, on average, by a set of physical, chemical, or biological processes, called “sinks,” that tend to remove CO2 from the atmosphere. Significant natural sinks include terrestrial vegetation, which takes up CO2 during the process of photosynthesis.A number of oceanic processes also act as carbon sinks. One such process, called the “solubility pump,” involves the descent of surface sea water containing dissolved CO2. Another process, the “biological pump,” involves the uptake of dissolved CO2 by marine vegetation and phytoplankton (small, free-floating, photosynthetic organisms) living in the upper ocean or by other marine organisms that use CO2 to build skeletons and other structures made of calcium carbonate (CaCO3). As these organisms expire and fall to the ocean floor, the carbon they contain is transported downward and eventually buried at depth. A long-term balance between these natural sources and sinks leads to the background, or natural, level of CO2 in the atmosphere.

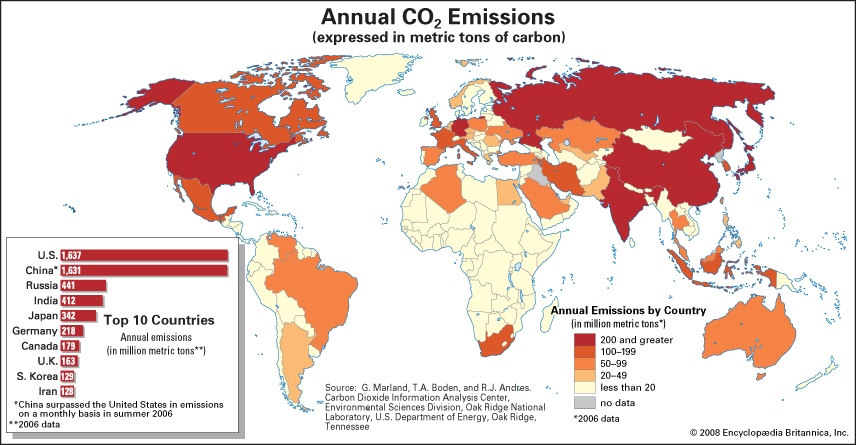
 In contrast, human activities increase atmospheric CO2 levels primarily through the burning of fossil fuels (fossil fuel) (principally oil (petroleum) and coal, and secondarily natural gas, for use in transportation, heating, and the production of electricity) and through the production of cement. Other anthropogenic sources include the burning of forests and the clearing of land. Anthropogenic emissions currently account for the annual release of about 7 gigatons (7 billion tons) of carbon into the atmosphere. Anthropogenic emissions are equal to approximately 3 percent of the total emissions of CO2 by natural sources, and this amplified carbon load from human activities far exceeds the offsetting capacity of natural sinks (by perhaps as much as 2–3 gigatons per year). CO2 has consequently accumulated in the atmosphere at an average rate of 1.4 parts per million (ppm) by volume per year between 1959 and 2006, and this rate of accumulation has been linear (that is, uniform over time). However, certain current sinks, such as the oceans, could become sources in the future. This may lead to a situation in which the concentration of atmospheric CO2 builds at an exponential rate.
In contrast, human activities increase atmospheric CO2 levels primarily through the burning of fossil fuels (fossil fuel) (principally oil (petroleum) and coal, and secondarily natural gas, for use in transportation, heating, and the production of electricity) and through the production of cement. Other anthropogenic sources include the burning of forests and the clearing of land. Anthropogenic emissions currently account for the annual release of about 7 gigatons (7 billion tons) of carbon into the atmosphere. Anthropogenic emissions are equal to approximately 3 percent of the total emissions of CO2 by natural sources, and this amplified carbon load from human activities far exceeds the offsetting capacity of natural sinks (by perhaps as much as 2–3 gigatons per year). CO2 has consequently accumulated in the atmosphere at an average rate of 1.4 parts per million (ppm) by volume per year between 1959 and 2006, and this rate of accumulation has been linear (that is, uniform over time). However, certain current sinks, such as the oceans, could become sources in the future. This may lead to a situation in which the concentration of atmospheric CO2 builds at an exponential rate.The natural background level of carbon dioxide varies on timescales of millions of years due to slow changes in outgassing through volcanic activity. For example, roughly 100 million years ago, during the Cretaceous Period, CO2 concentrations appear to have been several times higher than today (perhaps close to 2,000 ppm). Over the past 700,000 years, CO2 concentrations have varied over a far smaller range (between roughly 180 and 300 ppm) in association with the same Earth orbital effects linked to the coming and going of the ice ages (ice age) of the Pleistocene Epoch. By the early 21st century, CO2 levels reached 384 ppm, which is approximately 37 percent above the natural background level of roughly 280 ppm that existed at the beginning of the Industrial Revolution. According to ice core measurements, this level (384 ppm) is believed to be the highest in at least 650,000 years.
Radiative forcing caused by carbon dioxide varies in an approximately logarithmic fashion with the concentration of that gas in the atmosphere. The logarithmic relationship occurs as the result of a saturation effect wherein it becomes increasingly difficult, as CO2 concentrations increase, for additional CO2 molecules to further influence the “infrared window” (a certain narrow band of wavelengths in the infrared region that is not absorbed by atmospheric gases). The logarithmic relationship predicts that the surface warming potential will rise by roughly the same amount for each doubling of CO2 concentration. At current rates of fossil-fuel use, a doubling of CO2 concentrations over preindustrial levels is expected to take place by the middle of the 21st century (when CO2 concentrations are projected to reach 560 ppm). A doubling of CO2 concentrations would represent an increase of roughly 4 watts per square metre of radiative forcing. Given typical estimates of “climate sensitivity” in the absence of any offsetting factors, this energy increase would lead to a warming of 2 to 5 °C (3.6 to 9 °F) over preindustrial times. The total radiative forcing by anthropogenic CO2 emissions since the beginning of the industrial age is approximately 1.66 watts per square metre.
Methane
 methane (CH4) is the second most important greenhouse gas. CH4 is more potent than CO2 because the radiative forcing produced per molecule is greater. In addition, the infrared window is less saturated in the range of wavelengths of radiation absorbed by CH4, so more molecules may fill in the region. However, CH4 exists in far lower concentrations than CO2 in the atmosphere, and its concentrations by volume in the atmosphere are generally measured in parts per billion (ppb) rather than ppm. CH4 also has a considerably shorter residence time in the atmosphere than CO2 (the residence time for CH4 is roughly 10 years, compared with hundreds of years for CO2).
methane (CH4) is the second most important greenhouse gas. CH4 is more potent than CO2 because the radiative forcing produced per molecule is greater. In addition, the infrared window is less saturated in the range of wavelengths of radiation absorbed by CH4, so more molecules may fill in the region. However, CH4 exists in far lower concentrations than CO2 in the atmosphere, and its concentrations by volume in the atmosphere are generally measured in parts per billion (ppb) rather than ppm. CH4 also has a considerably shorter residence time in the atmosphere than CO2 (the residence time for CH4 is roughly 10 years, compared with hundreds of years for CO2).Natural sources of methane include tropical and northern wetlands (wetland), methane-oxidizing bacteria that feed on organic material consumed by termites (termite), volcanoes, seepage vents of the seafloor in regions rich with organic sediment, and methane hydrates trapped along the continental shelves (continental shelf) of the oceans and in polar permafrost. The primary natural sink for methane is the atmosphere itself, as methane reacts readily with the hydroxyl radical (OH-) within the troposphere to form CO2 and water vapour (H2O). When CH4 reaches the stratosphere, it is destroyed. Another natural sink is soil, where methane is oxidized (oxidation–reduction reaction) by bacteria.
As with CO2, human activity is increasing the CH4 concentration faster than it can be offset by natural sinks. Anthropogenic sources currently account for approximately 70 percent of total annual emissions, leading to substantial increases in concentration over time. The major anthropogenic sources of atmospheric CH4 are rice cultivation, livestock farming, the burning of coal and natural gas, the combustion of biomass, and the decomposition of organic matter in landfills. Future trends are particularly difficult to anticipate. This is in part due to an incomplete understanding of the climate feedbacks associated with CH4 emissions. In addition, as human populations grow, it is difficult to predict how possible changes in livestock raising, rice cultivation, and energy utilization will influence CH4 emissions.
It is believed that a sudden increase in the concentration of methane in the atmosphere was responsible for a warming event that raised average global temperatures by 4–8 °C (7.2–14.4 °F) over a few thousand years during the so-called Paleocene-Eocene Thermal Maximum, or PETM. This episode took place roughly 55 million years ago, and the rise in CH4 appears to have been related to a massive volcanic eruption that interacted with methane-containing flood deposits. As a result, large amounts of gaseous CH4 were injected into the atmosphere. It is difficult to know precisely how high these concentrations were or how long they persisted. At very high concentrations, residence times of CH4 in the atmosphere can become much greater than the nominal 10-year residence time that applies today. Nevertheless, it is likely that these concentrations reached several ppm during the PETM.
Methane concentrations have also varied over a smaller range (between roughly 350 and 800 ppb) in association with the Pleistocene ice age cycles. Preindustrial levels of CH4 in the atmosphere were approximately 700 ppb, whereas early 21st-century levels exceeded 1,770 ppb. (These concentrations are well above the natural levels observed for at least the past 650,000 years.) The net radiative forcing by anthropogenic CH4 emissions is approximately 0.5 watt per square metre—or roughly one-third the radiative forcing of CO2.
Lesser greenhouse gases
Surface-level ozone
 The next most significant greenhouse gas is surface, or low-level, ozone (O3). Surface O3 is a result of air pollution; it must be distinguished from naturally occurring stratospheric O3, which has a very different role in the planetary radiation balance. The primary natural source of surface O3 is the subsidence of stratospheric O3 from the upper atmosphere. In contrast, the primary anthropogenic source of surface O3 is photochemical reactions involving the atmospheric pollutant carbon monoxide (CO). The best estimates of the concentration of surface O3 are 50 ppb, and the net radiative forcing due to anthropogenic emissions of surface O3 is approximately 0.35 watt per square metre.
The next most significant greenhouse gas is surface, or low-level, ozone (O3). Surface O3 is a result of air pollution; it must be distinguished from naturally occurring stratospheric O3, which has a very different role in the planetary radiation balance. The primary natural source of surface O3 is the subsidence of stratospheric O3 from the upper atmosphere. In contrast, the primary anthropogenic source of surface O3 is photochemical reactions involving the atmospheric pollutant carbon monoxide (CO). The best estimates of the concentration of surface O3 are 50 ppb, and the net radiative forcing due to anthropogenic emissions of surface O3 is approximately 0.35 watt per square metre.Nitrous oxides and fluorinated gases
Additional trace gases produced by industrial activity that have greenhouse properties include nitrous oxide (N2O) and fluorinated gases (halocarbons (halocarbon)), the latter including sulfur hexafluoride, hydrofluorocarbons (HFCs), and perfluorocarbons (PFCs). Nitrous oxide is responsible for 0.16 watt per square metre radiative forcing, while fluorinated gases are collectively responsible for 0.34 watt per square metre. Nitrous oxides have small background concentrations due to natural biological reactions in soil and water, whereas the fluorinated gases owe their existence almost entirely to industrial sources.
- Ryan, T. Claude
- Ryan, Thomas Fortune
- rya rug
- Ryazan
- Rybakov, Anatoly
- Rybinsk
- Rybinsk Reservoir
- Rybnik
- Ry Cooder
- Rydberg constant
- Rydberg, Johannes Robert
- Rydberg, Viktor
- Ryde
- Ryder, Albert Pinkham
- Ryder Cup
- Ryder Cup Table
- Rye
- rye
- Ryedale
- ryegrass
- Rye House Plot
- Ryerson, Egerton
- rye whiskey
- Rykov, Aleksey Ivanovich
- Ryle, Gilbert