ytterbium
chemical element
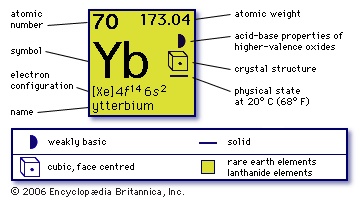 (Yb), chemical element, rare-earth metal of the lanthanoid series of the periodic table, a low-melting-point, divalent rare earth with little commercial use.
(Yb), chemical element, rare-earth metal of the lanthanoid series of the periodic table, a low-melting-point, divalent rare earth with little commercial use.The first concentrate of ytterbium was obtained (1878) by Jean-Charles-Galinard de Marignac (Marignac, Jean-Charles-Galinard de) and named by him for the town of Ytterby, Sweden, where the first rare-earth mineral was found. Georges Urbain and Carl Auer von Welsbach (Welsbach, Carl Auer, Freiherr von) independently demonstrated (1907–08) that Marignac's earth was composed of two oxides, which Urbain called neoytterbia and lutetia. The metals are now known as ytterbium and lutetium. Ytterbium is among the less-abundant rare earths. It occurs in minute amounts in many rare-earth minerals such as xenotime and euxenite and is found in the products of nuclear fission as well. Natural ytterbium consists of seven stable isotopes: ytterbium-168 (0.135 percent), ytterbium-170 (3.03 percent), ytterbium-171 (14.31 percent), ytterbium-172 (21.82 percent), ytterbium-173 (16.13 percent), ytterbium-174 (31.84 percent), and ytterbium-176 (12.73 percent).
Ytterbium may be separated from the other rare-earth elements by ion-exchange techniques. The elemental metal is prepared by the thermoreduction of its oxide, Yb2O3, with lanthanum metal. A compound of Yb in the +2 oxidation state was first prepared (1929) by W.K. Klemm and W. Schuth, who reduced ytterbium trichloride, YbCl3, to ytterbium dichloride, YbCl2, with hydrogen. The ion Yb2+ has also been produced by electrolytic reduction or treatment of a Yb3+ salt with sodium amalgam. The element forms a series of pale-green Yb2+ salts such as ytterbium sulfate, dibromide, hydroxide, and carbonate. The pale-green ytterbium ion Yb2+ is unstable in aqueous solution and reduces water readily, liberating hydrogen; it is less stable than the comparable europium ion, Eu2+, and more stable than the samarium ion Sm2+. In its predominant +3 oxidation state, ytterbium forms a series of white salts including the trioxide, the trisulfate, and the trinitrate.
The relatively soft, silvery-white metal is most conveniently prepared by thermoreduction of the oxide with lanthanum metal followed by distillation of the comparatively volatile ytterbium metal. Elemental ytterbium is oxidized slowly by air at room temperature; and it reacts with water, liberating hydrogen.
atomic number
70
atomic weight
173.04
melting point
824° C
boiling point
1,193° C
specific gravity
6.972 (25° C)
oxidation states
+2, +3
electronic config.
【Xe】4f 145d06s2
- Mediaş
- medical association
- medical education
- medical jurisprudence
- Medicare and Medicaid
- Medicean-Laurentian Library
- Medici Chapel
- Medici, Cosimo de'
- Medici Family
- Medici, Giovanni de'
- Medici, Giuliano de', Duc De Nemours
- Medici, Ippolito de'
- Medici, Lorenzino de'
- Medici, Lorenzo de'
- Medici, Lorenzo di Piero de', Duca Di Urbino
- medicinal leech
- medicinal poisoning
- medicine
- Medicine Bow Mountains
- Medicine Hat
- medicine, history of
- Medicine Lodge
- medicine man
- medicine society
- Medici, Piero di Cosimo de'