holmium
chemical element
 (Ho), chemical element, rare-earth metal of the lanthanoid series of the periodic table, one of the most paramagnetic substances known. Among the least abundant of the rare-earth elements, holmium and its compounds have limited application except for research. Holmium has been used as a component of some electronic devices; the ion Ho3+ has been used as a catalyst for ortho-para hydrogen conversion; and the oxide has been used as a special refractory.
(Ho), chemical element, rare-earth metal of the lanthanoid series of the periodic table, one of the most paramagnetic substances known. Among the least abundant of the rare-earth elements, holmium and its compounds have limited application except for research. Holmium has been used as a component of some electronic devices; the ion Ho3+ has been used as a catalyst for ortho-para hydrogen conversion; and the oxide has been used as a special refractory.Holmium was discovered (1878) spectroscopically by Jacques-Louis Soret and Marc Delafontaine and independently (1879) by Per Teodor Cleve (Cleve, Per Teodor), who separated it chemically from erbium and thulium. Cleve named the element for his native city of Stockholm, Sweden, its Latinized name being Holmia. Holmium occurs associated with other rare earths in the minerals xenotime, euxenite, and many others; it also occurs in the products of nuclear fission. The classical methods of separating and purifying the element were fractional crystallization and precipitation, but ion-exchange technology has now made available kilogram quantities of highly pure holmium oxide. The silvery metal is produced by thermoreduction of the anhydrous fluoride HoF3 with calcium. It is reactive and slowly attacked by oxygen and water. The one naturally occurring isotope, holmium-165, is stable; about two dozen radioactive artificial species are known.
Holmium behaves as a typical rare earth; it forms a series of yellow-brown salts, many of which are obtained in solution by dissolving the oxide Ho2O3 in the appropriate acid.
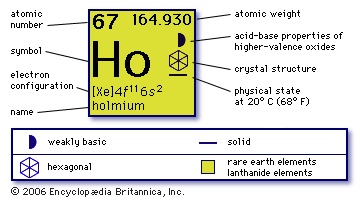
atomic number
67
atomic weight
164.930
melting point
1,470° C
boiling point
2,720° C
specific gravity
8.781 (25° C)
oxidation state
+3
electronic config.
【Xe】4f115d06s2
- Winifred Holt
- Winifred Sweet Black
- Winisk River
- Winkelman, Henri Gerard
- Winkfield, James
- Winkler, Clemens Alexander
- Winkler, Hans Günter
- Winkler Prins Encyclopedie
- Winneba
- Winnemucca
- Winnemucca, Sarah
- Winnetka
- Winnetka Plan
- Winnie Madikizela-Mandela
- Winnipeg
- Winnipeg Free Press
- Winnipeg, Lake
- Winnipegosis, Lake
- Winnipeg River
- Winnipesaukee, Lake
- Winogradsky, Sergey Nikolayevich
- Winold Reiss
- Winona
- Winona State University
- Winooski