hydrate
chemical compound
 any compound containing water in the form of H2O molecules (molecule), usually, but not always, with a definite content of water by weight. The best-known hydrates are crystalline solids that lose their fundamental structures upon removal of the bound water. Exceptions to this are the zeolites (zeolite) (aluminum silicate minerals (silicate mineral) or their synthetic analogues that contain water in indefinite amounts) as well as similar clay minerals (clay mineral), certain clays, and metallic oxides (oxide), which have variable proportions of water in their hydrated forms; zeolites lose and regain water reversibly with little or no change in structure.
any compound containing water in the form of H2O molecules (molecule), usually, but not always, with a definite content of water by weight. The best-known hydrates are crystalline solids that lose their fundamental structures upon removal of the bound water. Exceptions to this are the zeolites (zeolite) (aluminum silicate minerals (silicate mineral) or their synthetic analogues that contain water in indefinite amounts) as well as similar clay minerals (clay mineral), certain clays, and metallic oxides (oxide), which have variable proportions of water in their hydrated forms; zeolites lose and regain water reversibly with little or no change in structure.Substances that spontaneously absorb water from the air to form hydrates are known as hygroscopic or deliquescent, whereas hydrates that lose so-called water of hydration or water of crystallization to form the unhydrated (anhydrous) substances are known as efflorescent. In many cases, the uptake and loss of water (by heating, decreasing pressure, or other means) are reversible processes, sometimes accompanied by changes in colour. For example, blue vitriol, or copper sulfate pentahydrate (CuSO4∙5H2O), is blue, copper sulfate trihydrate (CuSO4∙3H2O) is blue, and anhydrous copper sulfate (CuSO4) is white.
Other examples of hydrates are Glauber's salt (sodium sulfate decahydrate, Na2SO4∙10H2O); washing soda (sodium carbonate decahydrate, Na2CO3∙10H2O); borax (sodium tetraborate decahydrate, Na2B4O7∙10H2O); the sulfates known as vitriols (vitriol) (e.g., Epsom salt, MgSO4∙7H2O); and the double salts known collectively as alums (M+2SO4∙M+32(SO4)3∙24H2O, where M+ is a monopositive cation, such as K+or NH4+, and M3+ is a tripositive cation, such as Al3+ or Cr3+).
In many cases, hydrates are coordination compounds (coordination compound). CuSO4∙5H2O is actually 【Cu(H2O) 4】SO4∙4H2O; four molecules of water of hydration are coordinated to the copper ion, whereas the fifth water molecule is linked to the sulfate ion, presumably by hydrogen bonding. Similarly, MgSO4∙7H2O is actually 【Mg(H2O) 6】SO4∙4H2O. X-ray diffraction studies have shown that hydrated beryllium sulfate (BeSO4∙4H2O) and hydrated beryllium nitrate (Be(NO3)2∙4H2O) both contain the tetrahedral complex ion 【Be(H2O) 4】4+.
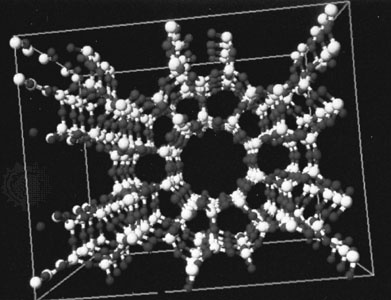
A number of gases—notably the noble gases (noble gas) and simple hydrocarbon gases such as methane, ethane, propane, and acetylene, as well as chlorine and carbon dioxide—form crystalline hydrates called clathrate compounds at relatively low temperatures and pressures. Clathrate crystals have a structure in which the water molecules form a loosely held framework surrounding the gas molecule.
Additional Reading
The chemistry of hydrates is covered in Mary Martinette Hagan, Clathrate Inclusion Compounds (1962); Jon A. McCleverty and Thomas Meyer (eds.), Comprehensive Coordination Chemistry II: From Biology to Nanotechnology, 10 vol. (2003); and Robyn V. Young and Suzanne Sessine (eds.), World of Chemistry (2000).
- Leavenworth
- Leavis, F.R.
- Leavitt, Henrietta Swan
- Lebachia
- Lebanon
- Lebanon, flag of
- Lebanon Mountains
- Lebedev, Pyotr Nikolayevich
- Lebedev, Sergey Vasilyevich
- Le Bel, Joseph-Achille
- Lebesgue, Henri-Léon
- Lebesgue integral
- Leblanc, Maurice
- Leblanc, Nicolas
- Le Blond, Alexandre-Jean-Baptiste
- Le Blon, Jakob Christof
- Leboeuf, Edmond
- Lebombo Mountains
- Le Bon, Gustave
- Lebon, Philippe
- Lebowa
- Lebowakgomo
- Lebrija
- Lebrun, Albert
- Le Brun, Charles