igneous rock
geology
Introduction
any of various crystalline or glassy rocks formed by the cooling and solidification of molten earth material. Igneous rocks comprise one of the three principal classes of rocks, the others being metamorphic and sedimentary.
Igneous rocks are formed from the solidification of magma, which is a hot (600° to 1,300° C, or 1,100° to 2,400° F) molten or partially molten rock material. The Earth is composed predominantly of a large mass of igneous rock with a very thin veneer of weathered material—namely, sedimentary rock. Whereas sedimentary rocks are produced by processes operating mainly at the Earth's surface by the disintegration of mostly older igneous rocks, igneous—and metamorphic—rocks are formed by internal processes that cannot be directly observed and that necessitate the use of physical-chemical arguments to deduce their origins. Because of the high temperatures within the Earth, the principles of chemical equilibrium are applicable to the study of igneous and metamorphic rocks, with the latter being restricted to those rocks formed without the direct involvement of magma.
Magma is thought to be generated within the plastic asthenosphere (the layer of partially molten rock underlying the Earth's crust) at a depth below about 60 kilometres (40 miles). Because magma is less dense than the surrounding solid rocks, it rises toward the surface. It may settle within the crust or erupt at the surface from a volcano as a lava flow. Rocks formed from the cooling and solidification of magma deep within the crust are distinct from those erupted at the surface mainly owing to the differences in physical and chemical conditions prevalent in the two environments. Within the Earth's deep crust the temperatures and pressures are much higher than at its surface; consequently, the hot magma cools slowly and crystallizes completely, leaving no trace of the liquid magma. The slow cooling promotes the growth of minerals large enough to be identified visually without the aid of a microscope (called phaneritic, from the Greek phaneros, meaning “visible”). On the other hand, magma erupted at the surface is chilled so quickly that the individual minerals have little or no chance to grow. As a result, the rock is either composed of minerals that can be seen only with the aid of a microscope (called aphanitic, from the Greek aphanēs, meaning “invisible”) or contains no minerals at all (in the latter case, the rock is composed of glass, which is a highly viscous liquid). This results in two groups: (1) plutonic intrusive igneous rocks that solidified deep within the crust and (2) volcanic, or extrusive, igneous rocks formed at the Earth's surface. Some intrusive rocks, known as subvolcanic, were not formed at great depth but were instead injected near the surface where lower temperatures result in a more rapid cooling process; these tend to be aphanitic and are referred to as hypabyssal intrusive rocks.
The deep-seated plutonic rocks can be exposed at the surface for study only after a long period of denudation or by some tectonic forces that push the crust upward or by a combination of the two conditions. (Denudation is the wearing away of the terrestrial surface by processes including weathering and erosion.) Generally, the intrusive rocks have cross-cutting contacts with the country rocks that they have invaded, and in many cases the country rocks show evidence of having been baked and thermally metamorphosed at these contacts. The exposed intrusive rocks are found in a variety of sizes, from small veinlike injections to massive dome-shaped batholiths, which extend for more than 100 square kilometres (40 square miles) and make up the cores of the great mountain ranges.
Extrusive rocks occur in two forms: (1) as lava flows that flood the land surface much like a river and (2) as fragmented pieces of magma of various sizes (pyroclastic materials), which often are blown through the atmosphere and blanket the Earth's surface upon settling. The coarser pyroclastic materials accumulate around the erupting volcano, but the finest pyroclasts can be found as thin layers located hundreds of kilometres from the opening. Most lava flows do not travel far from the volcano, but some low-viscosity flows that erupted from long fissures have accumulated in thick (hundreds of metres) sequences, forming the great plateaus of the world (e.g., the Columbia River plateau of Washington and Oregon and the Deccan Plateau in India). Both intrusive and extrusive magmas have played a vital role in the spreading of the ocean basin, in the formation of the oceanic crust, and in the formation of the continental margins. Igneous processes have been active since the formation of the Earth some 4.6 billion years ago. Their emanations have provided the water for the oceans, the gases for the primordial oxygen-free atmosphere, and many valuable mineral deposits.
Composition
Chemical components
The great majority of the igneous rocks are composed of silicate minerals (silicate mineral) (meaning that the basic building blocks for the magmas that formed them are made of silicon 【Si】 and oxygen 【O】), but minor occurrences of carbonate-rich (carbonate mineral) igneous rocks are found as well. Indeed, in 1960 a sodium carbonate (Na2CO3) lava with only 0.05 weight percent silica (SiO2) was erupted from Ol Doinyo Lengai, a volcano in northern Tanzania, Africa. Because of the limited occurrence of such carbonate-rich igneous rocks, however, the following discussion will consider the chemistry of silicate rocks only. The major oxides (oxide) of the rocks generally correlate well with their silica content: those rocks with low silica content are enriched in magnesium oxide (MgO) and iron oxides (FeO, Fe2O3, and Fe3O4) and are depleted in soda (Na2O) and potash (K2O); those with a large amount of silica are depleted in magnesium oxide and iron oxides but are enriched in soda and potash. Both calcium oxide (CaO) and alumina (Al2O3) are depleted in the rocks that have a silica content of less than about 45 weight percent, but, above 45 percent, calcium oxide can be as high as 10 percent; this amount decreases gradually as the silica increases. Alumina in rocks that contain more than 45 percent silica is generally above approximately 14 weight percent, with the greatest abundance occurring at an intermediate silica content of about 56 weight percent. Because of the importance of silica content, it has become common practice to use this feature of igneous rocks as a basis for subdividing them into the following groups: silicic or felsic (felsic and mafic rocks) (or acid, an old and discredited but unfortunately entrenched term), rocks having more than 66 percent silica; intermediate, rocks with 55 to 66 percent silica; and subsilicic, rocks containing less than 55 percent silica. The latter may be further divided into two groups: mafic (mafic rock), rocks with 45 to 55 percent silica and ultramafic, those containing less than 45 percent. The subsilicic rocks, enriched as they are in iron (Fe) and magnesium (Mg), are termed femic (from ferrous iron and magnesium), whereas the silicic rocks are referred to as sialic (from silica and aluminum, with which they are enriched) or salic (from silica and aluminum). The terms mafic (from magnesium and ferrous iron) and felsic (feldspar and silica) are used interchangeably with femic and sialic.
The silica content also reflects the mineral composition of the rocks. As the magma cools and begins to crystallize, silica is taken from the magma to be combined with the other cationic oxides to form the silicate minerals. For example, one mole of SiO2 is combined with one mole of MgO to make the magnesium-rich pyroxene, MgSiO3 ( enstatite): SiO2 + MgO → MgSiO3. Two moles of SiO2 are needed to be combined with one mole each of CaO and Al2O3 to make the calcium-rich plagioclase, CaAl2Si2O8 ( anorthite). However, in a case where magma does not have enough silica relative to the magnesium oxide to produce the pyroxene, the magma will compensate by making a magnesium-olivine (forsterite; Mg2SiO4), along with the pyroxene, since the olivine requires only one-half as much silica for every mole of magnesium oxide. On the other hand, a silicic magma may have excess silica such that some will be left after all the silicate minerals were formed from the combination of the oxides; the remaining “free” silica crystallizes as quartz or its polymorphs. The former case usually occurs in subsilicic rocks that characteristically will have silicate minerals like magnesium-olivine, sodium- nepheline (NaAlSiO4, which requires only one mole of silicon for every mole of sodium 【Na】), and leucite (KAlSi2O6, which requires only two moles of silicon to one mole of potassium 【K】). These three minerals substitute in part for enstatite, albite (NaAlSi3O8, requiring three moles of silicon for one mole of sodium), and orthoclase feldspar (KAlSi3O8, requiring three moles of silicon for one mole of potassium), respectively. Quartz clearly will not be present in these rocks. Minerals such as magnesium-olivine, nepheline, and leucite are termed undersaturated (with respect to silica), and the subsilicic rocks that contain them are termed undersaturated as well. In the case of rocks that have excess silica, the silicic rocks will have quartz and magnesium-pyroxene, which are considered saturated minerals, and the rocks that contain them are termed supersaturated.
Mineralogical components
The major mineralogical components of igneous rocks can be divided into two groups: felsic (from feldspar and silica) and mafic (from magnesium and ferrous iron). The felsic minerals include quartz, tridymite, cristobalite, feldspars (plagioclase and alkali feldspar), feldspathoids (nepheline and leucite), muscovite, and corundum. Because felsic minerals lack iron and magnesium, they are generally light in colour and consequently are referred to as such or as leucocratic. The mafic minerals include olivine, pyroxenes, amphiboles, and biotites, all of which are dark in colour. Mafic minerals are said to be melanocratic. These terms can be applied to the rocks, depending on the relative proportion of each type of mineral present. In this regard, the term colour index, which refers to the total percentage of the rock occupied by mafic minerals, is useful. Felsic rocks have a colour index of less than 50, while mafic rocks have a colour index above 50. Those rocks that have a colour index above 90 are referred to as ultramafic. These terms are to be used only for the mineralogical content of igneous rocks because they do not necessarily correlate directly with chemical terms. For example, it is common to find a felsic rock composed almost entirely of the mineral plagioclase, but in chemical terms, such a rock is a subsilicic mafic rock. Another example is an igneous rock consisting solely of pyroxene. Mineralogically it would be termed ultramafic, but chemically it is a mafic igneous rock with a silica content of about 50 percent.
The influence of supersaturation and undersaturation on the mineralogy of a rock was noted above. During the crystallization of magmas, supersaturated minerals will not be formed along with undersaturated minerals. Supersaturated minerals include quartz and its polymorphs and a low-calcium orthorhombic pyroxene. These cannot coexist with any of the feldspathoids (e.g., leucite and nepheline) or magnesium-rich olivine. In volcanic rocks that have been quenched (cooled rapidly) such that only a small part of the magma has been crystallized, it is possible to find a forsterite (magnesium-rich olivine) crystal surrounded by a glass that is saturated or supersaturated. In this case, the outer rim of the olivine may be corroded or replaced by a magnesium-rich pyroxene (called a reaction rim). The olivine was the first to be crystallized, but it was in the process of reacting with the saturated magma to form the saturated mineral when an eruption halted the reaction. Had the magma been allowed to crystallize fully, all the forsterite would have been transformed into the magnesium-rich pyroxene and quartz may have been crystallized.
Accessory minerals present in igneous rocks in minor amounts include monazite, allanite, apatite, garnets, ilmenite, magnetite, titanite, spinel, and zircon. Glass may be a major phase in some volcanic rock but, when present, is usually found in minor amounts. Igneous rocks that were exposed to weathering and circulating groundwater have undergone some degree of alteration. Common alteration products are talc or serpentine formed at the expense of olivine, chlorites replacing pyroxene and amphiboles, iron oxides replacing any mafic mineral, clay minerals and epidote formed from the feldspars, and calcite that may be formed at the expense of any calcium-bearing mineral by interaction with a carbon dioxide (CO2)-bearing solution. Glass is commonly altered to clay minerals and zeolite. In some cases, however, glass has undergone a devitrification process (in which it is transformed into a crystalline material) initiated by reaction of the glass with water or by subsequent reheating. Common products of devitrification include quartz and its polymorphs, alkali feldspar, plagioclase, pyroxene, zeolite, clays, and chlorite.
Textural features
The texture of an igneous rock normally is defined by the size and form of its constituent mineral grains and by the spatial relationships of individual grains with one another and with any glass that may be present. Texture can be described independently of the entire rock mass, and its geometric characteristics provide valuable insights into the conditions under which the rock was formed.
Crystallinity
Crystallinity categories of igneous rocksAmong the most fundamental properties of igneous rocks are crystallinity and granularity, two terms that closely reflect differences in magma composition and the differences between volcanic and various plutonic environments of formation. Crystallinity generally is described in terms of the four categories shown in the Table (Crystallinity categories of igneous rocks).
Those holocrystalline rocks in which mineral grains can be recognized with the unaided eye are called phanerites, and their texture is called phaneritic. Those with mineral grains so small that their outlines cannot be resolved without the aid of a hand lens or microscope are termed aphanites, and their texture is termed aphanitic. Aphanitic rocks are further described as either microcrystalline or cryptocrystalline, according to whether or not their individual constituents can be resolved under the microscope. The subaphanitic, or hyaline, rocks are referred to as glassy, or vitric, in terms of granularity.
Categories of rock grain sizeAphanitic and glassy textures represent relatively rapid cooling of magma and, hence, are found mainly among the volcanic rocks. Slower cooling, either beneath the Earth's surface or within very thick masses of lava, promotes the formation of crystals and, under favourable circumstances of magma composition and other factors, their growth to relatively large sizes. The resulting phaneritic rocks are so widespread and so varied that it is convenient to specify their grain size as shown in the Table (Categories of rock grain size).
Granularity
Grain size
The general grain size ordinarily is taken as the average diameter of dominant grains in the rock; for the pegmatites, which are special rocks with extremely large crystals, it can refer to the maximum exposed dimensions of dominant grains. Most aphanitic rocks are characterized by mineral grains less than 0.3 millimetre (0.01 inch) in diameter, and those in which the average grain size is less than 0.1 millimetre (0.004 inch) are commonly described as dense.
Fabric
A major part of rock texture is fabric or pattern, which is a function of the form and outline of its constituent grains, their relative sizes, and their mutual relationships in space. Many specific terms have been employed to shorten the description of rock fabrics, and even the sampling offered here may seem alarmingly extensive. It should be noted, however, that fabric provides some of the most useful clues to the nature and sequence of magmatic crystallization.
The degree to which mineral grains show external crystal faces can be described as euhedral or panidiomorphic (fully crystal-faced), subhedral or hypidiomorphic (partly faced), or anhedral or allotriomorphic (no external crystal faces). Quite apart from the presence or absence of crystal faces, the shape, or habit, of individual mineral grains is described by such terms as equant, tabular, platy, elongate, fibrous, rodlike, lathlike, needlelike, and irregular. A more general contrast can be drawn between grains of equal (equant) and inequal dimensions. Even-grained, or equigranular, rocks are characterized by essential minerals that all exhibit the same order of grain size, but this implied equality need not be taken too literally. For such rocks the combination terms panidiomorphic-granular, hypidiomorphic-granular, and allotriomorphic-granular are applied according to the occurrence of euhedral, subhedral, and anhedral mineral grains within them. Many fine-grained allotriomorphic-granular rocks are more simply termed sugary, saccharoidal, or aplitic.
Rocks that are unevenly grained, or inequigranular, are generally characterized either by a seriate fabric, in which the variation in grain size is gradual and essentially continuous, or by a porphyritic fabric, involving more than one distinct range of grain sizes. Both of these kinds of texture are common. The relatively large crystals in a porphyritic rock ordinarily occur as separate entities, known as phenocrysts, set in a groundmass or matrix of much finer-grained crystalline material or glass. Quite commonly in many volcanic rocks, phenocrysts are aggregated. When this is observed, the term glomeroporphyritic is used to describe the texture, and the aggregate is referred to as a glomerocryst. In some cases, such glomerocrysts are monomineralic, but more commonly they are composed of two or more minerals. Based on chemical composition, texture, and other criteria such as isotopic analysis, it has been demonstrated that some phenocrysts and glomerocrysts were not crystallized from the host magma but rather were accidentally torn from the country rock by the magma as it rose to the surface. When this has occurred, these phenocrysts are referred to as xenocrysts, while the aggregates can be termed xenoliths (xenolith). The size of phenocrysts is essentially independent of their abundance relative to the groundmass, and they range in external form from euhedral to anhedral. Most of them are best described as subhedral. Because the groundmass constituents span almost the full ranges of crystallinity and granularity, porphyritic fabric is abundantly represented among the phaneritic, aphanitic, and glassy rocks.
The sharp break in grain size between phenocrysts and groundmass reflects a corresponding change in the conditions that affected the crystallizing magma. Thus, the phenocrysts of many rocks probably grew slowly at depth, following which the nourishing magma rose to the Earth's surface as lava, cooled much more rapidly, and congealed to form a finer-grained or glassy groundmass. A porphyritic volcanic rock with a glassy groundmass is described as having a vitrophyric texture and the rock can be called a vitrophyre. Other porphyritic rocks may well reflect less drastic shifts in position and perhaps more subtle and complex changes in conditions of temperature, pressure, or crystallization rates. Many phenocrysts could have developed at the points where they now occur, and some may represent systems with two fluid phases, magma and coexisting gas. Appraisals of the composition of phenocrysts, their distribution, and their periods of growth relative to the accompanying groundmass constituents are important to an understanding of many igneous processes.
Important textural types
The articulation of mineral grains is described in terms of planar, smoothly curved, sinuous, sutured, interlocked, or irregular surfaces of mutual boundary. The distribution and orientation of mineral grains and of mineral grains and glass are other elements of fabric that can be useful in estimating the conditions and sequence of mineral formation in igneous rocks. The following are only a few of the most important examples:
Directive textures are produced by the preferred orientation of platy, tabular, or elongate mineral grains to yield grossly planar or linear arrangements; they are generally a result of magmatic flowage.
Graphic texture refers to the regular intergrowth of two minerals, one of them generally serving as a host and the other appearing on surfaces of the host as striplike or cuneiform units with grossly consistent orientation; the graphic intergrowth of quartz in alkali feldspar is a good example.
Ophitic texture is the association of lath-shaped euhedral crystals of plagioclase, grouped radially or in an irregular mesh, with surrounding or interstitial large anhedral crystals of pyroxene; it is characteristic of the common rock type known as diabase.
Poikilitic texture describes the occurrence of one mineral that is irregularly scattered as diversely oriented crystals within much larger host crystals of another mineral.
Reaction textures occur at the corroded margins of crystals, from the corrosive rimming of crystals of one mineral by finer-grained aggregates of another, or as a result of other features that indicate partial removal of crystalline material by reaction with magma or other fluid.
Pyroclastic texture results from the explosive fragmentation of volcanic material, including magma (commonly the light, frothy pumice variety and glass fragments called shards), country rock, and phenocrysts. Fragments less than 2 millimetres in size are called ash, and the rock formed of these is called tuff; (tuff) fragments between 2 and 64 millimetres are lapilli (lapillus) and the rock is lapillistone; fragments greater than 64 millimetres are called bombs (bomb) if rounded or blocks if angular, and the corresponding rock is termed agglomerate or pyroclastic breccia, respectively. Commonly, many of these pyroclastic rocks have been formed by dense hot clouds that hug the ground and behave much like a lava flow and hence are given the name pyroclastic flow. Most of these flows are composed of ash-size material; therefore, they are called ash flows and the rocks deposited by them are called ash-flow tuffs. A more general term for rocks deposited by these flows that does not specify size of fragments is ignimbrite. Ash-flow tuffs and other ignimbrites often have zones in which the fragments have been welded. These zones are termed welded tuffs (welded tuff) and display a directive planar texture (called eutaxitic) that results from compaction and flattening of pumice fragments. Such pyroclastic flows were responsible for many of the deposits of the eruption of Mount St. Helens in Washington State, U.S., on May 18, 1980. Most eruptions eject fragments that are borne by the wind and deposited subaerially (on the land surface). These deposits are said to be ash-fall tuffs and are recognized by their lamination (formation in thin layers that differ in grain size or composition). They commonly blanket the topography in contrast to the ash-flow deposits, which flow around topographic highs and which are completely unsorted.
Replacement textures occur where a mineral or mineral aggregate has the external crystal form of a preexisting different mineral (pseudomorphism (pseudomorph)) or where the juxtaposition of two minerals indicates that one was formed at the expense of the other.
Finally, crystal zoning describes faintly to very well-defined geometric arrangements of portions within individual crystals that differ significantly in composition (or some other property) from adjacent portions; most common are successive shells grouped concentrically about the centres of crystals, presumably reflecting shifts in conditions during crystal growth.
Structural features
The structure of an igneous rock is normally taken to comprise the mutual relationships of mineral or mineral-glass aggregates that have contrasting textures, along with layering, fractures, and other larger-scale features that transect or bound such aggregates. Structure often can be described only in relation to masses of rock larger than a hand specimen, and most of its individual expressions can be closely correlated with physical conditions that existed when the rock was formed.
Small-scale structural features
Among the most widespread structural features of volcanic rocks (extrusive rock) are the porelike openings left by the escape of gas from the congealing lava. Such openings are called vesicles, and the rocks in which they occur are said to be vesicular. Where the openings lie close together and form a large part of the containing rock, they impart to it a slaglike, or scoriaceous (scoria), structure. Their relative abundance is even greater in the type of sialic glassy rock known as pumice, which is essentially a congealed volcanic froth. Most vesicles can be likened to peas or nuts in their ranges of size and shape; those that were formed when the lava was still moving tend to be flattened and drawn out in the direction of flow. Others are cylindrical, pearlike, or more irregular in shape, depending in part on the manner of escape of the gas from the cooling lava; most of the elongate ones occur in subparallel arrangements.
Many vesicles have been partly or completely filled with quartz, chalcedony, opal, calcite, epidote, zeolites, or other minerals. These fillings are known as amygdules (amygdule), and the rock in which they are present is amygdaloidal. Some are concentrically layered, others also include centrally disposed series of horizontal layers, and still others are featured by central cavities into which well-formed crystals project.
Spherulites are light-coloured subspherical masses that commonly consist of tiny fibres and plates of alkali feldspar radiating outward from a centre. Most range from pinpoint to nut size, but some are as much as several feet in diameter. The relatively large ones tend to be internally complex and to contain concentric shells of feldspar fibres with or without accompanying quartz, tridymite, or glass. Spherulites occur mainly in glassy volcanic rocks; they also are present in some partly or wholly crystalline rocks that include shallow-seated intrusive types. Many evidently are products of rapid crystallization, perhaps at points of gas concentration in the freezing magmas. Others, in contrast, were formed more slowly, by devitrification of volcanic glasses, presumably not long after they congealed and while they were still relatively hot.
Lithophysae, also known as stone bubbles, consist of concentric shells of finely crystalline alkali feldspar separated by empty spaces; thus, they resemble an onion or a newly blooming rose. Commonly associated with spherulites in glassy and partly crystalline volcanic rocks of salic composition, many lithophysae are about the size of walnuts. They have been ascribed to short episodes of rapid crystallization, alternating with periods of gas escape when the open spaces were developed by thrusting the feldspathic shells apart or by contraction associated with cooling. The curving cavities commonly are lined with tiny crystals of quartz, tridymite, feldspar, topaz, or other minerals deposited from the gases.
Some glassy rocks of silicic composition are marked by domains of strongly curved, concentrically disposed fractures that promote breakage into rounded masses of pinhead to walnut size. Because their surfaces often have a pearly or shiny lustre, the name perlite is applied to such rocks. Perlite is most common in glassy silicic rocks that have interacted with water to become hydrated. During the hydration process, water enters the glass, breaking the silicon-oxygen bonds and causing an expansion of the glass structure to form the curved cracks. The extent of hydration of glass, indicating the amount of perlite that has been formed from the glass, depends on the climate and on time. In a given area where the climate is expected to be consistent, the thickness of the hydration of the glass surface has been used by archaeologists to date artifacts such as arrowheads composed of the dark volcanic glass known as obsidian and made by early native Americans.
Numerous structural features of comparably small scale occur among the intrusive rocks; these include miarolitic, orbicular, plumose, and radial structures. Miarolitic rocks are felsic phanerites distinguished by scattered pods or layers, ordinarily several centimetres in maximum thickness, within which their essential minerals are coarser-grained, subhedral to euhedral, and otherwise pegmatitic in texture. Many of these small interior bodies, called miaroles, contain centrally disposed crystal-lined cavities that are known as druses or miarolitic cavities. An internal zonal disposition of minerals also is common, and the most characteristic sequence is alkali feldspar with graphically intergrown quartz, alkali feldspar, and a central filling of quartz. Miarolitic structure probably represents local concentration of gases during very late stages in consolidation of the host rocks.
The term orbicular is applied to rounded, onionlike masses with distinct concentric layering that are distributed in various ways through otherwise normal-appearing phaneritic rocks of silicic to mafic composition. The layers within individual masses are typically thin, irregular, and sharply defined, and each differs from its immediate neighbours in composition or texture. Some layers contain tabular or prismatic mineral grains that are oriented radially with respect to the containing orbicule and, hence, are analogous to spherulitic layers in volcanic rocks. The minerals of most orbicules are the same as those of the enclosing rock, but they are not necessarily present in the same proportions. The concentric structure appears to reflect rhythmic crystallization about specific centres, commonly at early stages in consolidation of the general rock mass.
The normal fabric of some relatively coarse-grained plutonic rocks is interrupted by clusters of crystals with radial grouping but without concentric layering. A characteristic plumelike, spraylike, or rosettelike structure is imparted by the markedly elongate form of the participating crystals or crystal aggregates, which seem to have developed outward from common centres by direct crystallization from magma or by replacement of preexisting solid material.
Large-scale structural features
Many kinds of larger-scale features occur among both the intrusive and the extrusive rocks. Most of these are mentioned later in connection with rock occurrence or are discussed in other articles, but several are properly introduced here:
Clastic structures
These are various features that express the accumulation of fragments or the rupturing and dislocation of solid material. In volcanic environments they generally result from explosive activity or the incorporation of solid fragments by moving lava; as such, they characterize the pyroclastic rocks. Among the plutonic rocks, they appear chiefly as local to very extensive zones of pervasive shearing, dislocation, and granulation, commonly best recognized under the microscope. Those developed prior to final consolidation of the rock are termed protoclastic; those developed after final consolidation, cataclastic.
Flow structures
These are planar or linear features that result from flowage of magma with or without contained crystals. Various forms of faintly to sharply defined layering and lining typically reflect compositional or textural inhomogeneities, and they often are accentuated by concentrations or preferred orientation of crystals, inclusions, vesicles, spherulites, and other features.
Fractures
These are straight or curving surfaces of rupture directly associated with the formation of a rock or later superimposed upon it. Primary fractures generally can be related to emplacement or to subsequent cooling of the host rock mass. The columnar jointing found in many mafic volcanic rocks is a typical result of contraction upon cooling.
Inclusions
These are rounded to angular masses of solid material enclosed within a rock of recognizably different composition or texture. Those consisting of older material not directly related to that of their host are known as xenoliths (xenolith), and those representing broken-up and detached older parts of the same igneous body that encloses them are termed cognate xenoliths or autoliths.
Pillow structures
These are aggregates of ovoid masses, resembling pillows or grain-filled sacks in size and shape, that occur in many basic volcanic rocks. The masses are separated or interconnected, and each has a thick vesicular crust or a thinner and more dense glassy rind. The interiors ordinarily are coarser-grained and less vesicular. Pillow structure is formed by rapid chilling of highly fluid lava in contact with water or water-saturated sediments, accompanied by the development of budlike projections with tough, elastic crusts. As additional lava is fed into each bud, it grows into a pillow and continues to enlarge until rupture of the skin permits escape of fresh lava to form a new bud and a new pillow.
Segregations
These are special types of inclusions that are intimately related to their host rocks and in general are relatively rich in one or more of the host-rock minerals. They range from small pods to extensive layers and from early-stage crystal accumulations formed by gravitational settling in magma to very late-stage concentrations of coarse-grained material developed in place.
Zonal structures
These are arrangements of rock units with contrasting composition, or texture, in an igneous body, commonly in a broadly concentric pattern. Chilled margins, the fine-grained or glassy edges along the borders of many extrusive and shallow-seated intrusive bodies, represent quenching of magma along contacts with cooler country rock. Other kinds of zones generally reflect fractional crystallization of magma and are useful in tracing courses of magmatic differentiation, as will be noted later.
An interesting type of zonal structure is an orbicular configuration that has alternating light and dark repeating bands in an oval arrangement found in some diorites (diorite) and granodiorites. Pegmatites (pegmatite) also often have zonal structures due to fluctuations in fluid composition. This results in “pockets” that may contain gems or other unusual minerals.
Classification of igneous rocks
Igneous rocks are classified on the basis of mineralogy, chemistry, and texture. As discussed earlier, texture is used to subdivide igneous rocks into two major groups: (1) the plutonic rocks, with mineral grain sizes that are visible to the naked eye, and (2) the volcanic and hypabyssal types, which are usually too fine-grained or glassy for their mineral composition to be observed without the use of a petrographic microscope. Being rather coarsely grained, phaneritic rocks readily lend themselves to a classification based on mineralogy since their individual mineral components can be discerned, but the volcanic rocks are more difficult to classify because either their mineral composition is not visible or the rock has not fully crystallized owing to fast cooling. As a consequence, various methods employ chemical composition as the criterion for volcanic igneous rock classification. A commonly used technique was introduced at the beginning of the 20th century by the American geologists C. Whitman Cross, Joseph P. Iddings, Louis V. Pirsson, and Henry S. Washington. In this method, the mineral composition of the rock is recalculated into a standard set of typically occurring minerals that theoretically could have developed from the complete equilibrium crystallization at low temperatures of a magma of the indicated bulk composition. The calculated hypothetical mineral composition is called the norm, and the minerals constituting the standard set are termed normative minerals, since they are ordinarily found in igneous rocks. The rock under analysis may then be classified according to the calculated proportions of the normative minerals.
Because other methods for calculating the norm have been devised, this original norm is referred to as the CIPW norm after the initials of the four petrologists who devised the system. The norm calculation allows the petrologist studying an aphanitic rock to “see” the mineral assemblage that corresponds well with the actual mineral assemblage of a plutonic rock of the same composition that had crystallized under equilibrium conditions. Moreover, the norm has been shown to have a thermodynamic basis. The concept of silica saturation discussed above is incorporated into the norm, which will show whether a magma of a certain composition is supersaturated, saturated, or undersaturated by the presence or absence of normative minerals such as quartz, orthopyroxene, olivine, and the feldspathoids.
Classification of plutonic rocks
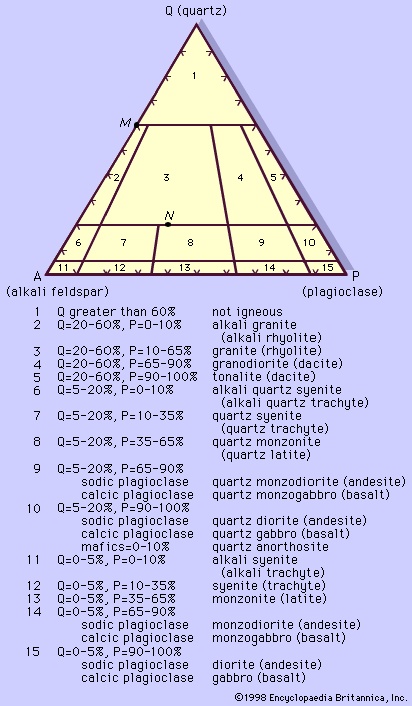 A plutonic rock may be classified mineralogically based on the actual proportion of the various minerals of which it is composed (called the mode). In any classification scheme, boundaries between classes are set arbitrarily; however, if the boundaries can be placed closest to natural divisions or gaps between classes, they will seem less random and subjective, and the standards will facilitate universal understanding. In order to set boundaries nearest to the population lows (of constituent minerals) and to achieve an international consensus, a poll among the world's petrologists was conducted and a modal classification for plutonic igneous rocks was devised. Based mainly on this poll, the International Union of Geological Sciences (IUGS) Subcommission on the Systematics of Igneous Rocks in 1973 suggested the use of the modal composition for all plutonic igneous rocks with a colour index less than 90 (Figure 1-->
A plutonic rock may be classified mineralogically based on the actual proportion of the various minerals of which it is composed (called the mode). In any classification scheme, boundaries between classes are set arbitrarily; however, if the boundaries can be placed closest to natural divisions or gaps between classes, they will seem less random and subjective, and the standards will facilitate universal understanding. In order to set boundaries nearest to the population lows (of constituent minerals) and to achieve an international consensus, a poll among the world's petrologists was conducted and a modal classification for plutonic igneous rocks was devised. Based mainly on this poll, the International Union of Geological Sciences (IUGS) Subcommission on the Systematics of Igneous Rocks in 1973 suggested the use of the modal composition for all plutonic igneous rocks with a colour index less than 90 (Figure 1--> ) and for those plutonic ultramafic rocks with a colour index greater than 90.
) and for those plutonic ultramafic rocks with a colour index greater than 90.

 The plotting of rock modes on these triangular diagrams is simpler than it may appear. If the colour index is less than 90 and quartz (Q) is present, then the three components, Q + A (alkali feldspar) + P (plagioclase), are recalculated from the mode to sum to 100 percent and Figure 1-->
The plotting of rock modes on these triangular diagrams is simpler than it may appear. If the colour index is less than 90 and quartz (Q) is present, then the three components, Q + A (alkali feldspar) + P (plagioclase), are recalculated from the mode to sum to 100 percent and Figure 1--> is used. Each component is represented by the corners of the equilateral triangle, the length of whose sides are divided into 100 equal parts. Any composition plotting at a corner, therefore, has a mode of 100 percent of the corresponding component. Any point on the sides of the triangle represents a mode composed of the two adjacent corner components. For example, a rock with 60 percent Q and 40 percent A will plot on the QA side at a location 60 percent of the distance from A to Q (see point M in Figure 1-->
is used. Each component is represented by the corners of the equilateral triangle, the length of whose sides are divided into 100 equal parts. Any composition plotting at a corner, therefore, has a mode of 100 percent of the corresponding component. Any point on the sides of the triangle represents a mode composed of the two adjacent corner components. For example, a rock with 60 percent Q and 40 percent A will plot on the QA side at a location 60 percent of the distance from A to Q (see point M in Figure 1--> ). A rock containing all three components will plot within the triangle. Since the sides of the triangle are divided into 100 parts, a rock having a mode of 20 percent Q and 80 percent A + P (in unknown proportions for the moment) will plot on the line that parallels the AP side and lies 20 percent of the distance toward Q from the side AP. If this same rock has 30 percent P and 50 percent A, the rock mode will plot at the intersection of the 20 percent Q line described above, with a line paralleling the QA side at a distance 30 percent toward P from the QA side (see point N in Figure 1-->
). A rock containing all three components will plot within the triangle. Since the sides of the triangle are divided into 100 parts, a rock having a mode of 20 percent Q and 80 percent A + P (in unknown proportions for the moment) will plot on the line that parallels the AP side and lies 20 percent of the distance toward Q from the side AP. If this same rock has 30 percent P and 50 percent A, the rock mode will plot at the intersection of the 20 percent Q line described above, with a line paralleling the QA side at a distance 30 percent toward P from the QA side (see point N in Figure 1--> ). The third intersecting line for the point is necessarily the line paralleling the QP side at 50 percent of the distance from the side QP toward A.
). The third intersecting line for the point is necessarily the line paralleling the QP side at 50 percent of the distance from the side QP toward A.A rock with 25 percent Q, 35 percent P, and 40 percent A plots in the granite field, whereas one with 25 percent Q, 60 percent P, and 15 percent A plots in the granodiorite field. The latter is close to the average composition of the continental crust of the Earth. Igneous rocks normally do not exceed about 50 percent quartz, and the feldspathoidal rocks are relatively rare. The most common plutonic rocks are those in fields numbered 3, 4, 5, 8, 9, 10, and 15. These are found in what have been called granite (used in a loose sense) batholiths (batholith), which are irregularly shaped large bodies covering an area greater than 100 square kilometres. Batholiths constitute the cores of the great mountain ranges, such as the Rockies in western North America and the Sierra Nevada in California, U.S. Typically these batholiths are composites of smaller intrusions, each of which may display several different rock types. The average composition is close to that of a granodiorite, but in many batholiths the sequence of intrusions progresses from basic to acidic, with gabbro or quartz diorite being emplaced first. In the Sierra Nevada batholith, the dominant rocks are quartz monzonite and granodiorite, with intrusions including quartz diorite in the far western rim and granite in the east. Batholiths contain medium- to coarse-grained rocks with hypidiomorphic-granular texture. The rocks are generally leucocratic; diorites and quartz diorites typically contain less than 30 percent mafic minerals—e.g., hornblende and biotite. Pyroxenes are rare but are more commonly found in the gabbros. Mineralogically the ratio of hornblende to biotite, the colour index, the calcium content of the plagioclase feldspar, and the ratio of plagioclase to alkali feldspar decrease from diorite to quartz diorite to granodiorite and granite. Common accessory minerals include apatite, titanite, and an opaque mineral such as magnetite or ilmenite.
 Ideally it would be preferable to use the same modal scheme for volcanic rocks. This is recommended whenever possible; hence, for this purpose, the volcanic or hypabyssal equivalent of the plutonic rocks are listed in parentheses in Figure 1-->
Ideally it would be preferable to use the same modal scheme for volcanic rocks. This is recommended whenever possible; hence, for this purpose, the volcanic or hypabyssal equivalent of the plutonic rocks are listed in parentheses in Figure 1--> . It should be noted that the dividing lines and boxes are identical to those for the plutonic rocks.
. It should be noted that the dividing lines and boxes are identical to those for the plutonic rocks.Classification of volcanic and hypabyssal rocks
Owing to the aphanitic texture of volcanic (extrusive rock) and hypabyssal rocks, their modes cannot be readily determined; consequently, a chemical classification is widely accepted and employed by most petrologists. One popular scheme is based on the use of both chemical components and normative mineralogy. Because most lay people have little access to analytic facilities that yield igneous rock compositions, only an outline will be presented here in order to provide an appreciation for the classification scheme.
The first major division is based on the alkali (soda + potash) and silica contents, which yield two groups, the subalkaline and alkaline rocks. The subalkaline rocks have two divisions based mainly on the iron content, with the iron-rich group called the tholeiitic series and the iron-poor group called calc-alkalic. The former group is most commonly found along the oceanic ridges and on the ocean floor; the latter group is characteristic of the volcanic regions of the continental margins (convergent, or destructive, plate boundaries; see below Forms of occurrence: Distribution of igneous rocks on the Earth's surface (igneous rock)). In some magmatic arcs (groups of islands arranged in a curved pattern), notably Japan, both the tholeiitic and calc-alkalic series occur. This is the case, for example, in the volcanoes of northeastern Honshu, the largest of Japan's four main islands, and both series may be found within the same volcano. The alkaline rocks frequently occur on oceanic islands (usually formed during the late stages of magma consolidation after tholeiitic eruptions) and in continental rifts (extensive fractures). Based on the relative proportions of soda and potash, the calc-alkalic series is subdivided into the sodic and potassic series.
Chemically the subalkaline rocks are saturated with respect to silica; consequently, they have normative minerals such as orthopyroxene 【Mg(Fe)2Si2O6】 and quartz but lack nepheline and olivine (in the presence of quartz). This chemical property also is reflected in the mode of the basic members that have two pyroxenes, orthopyroxene and augite 【Ca(Mg, Fe)Si2O6】, and perhaps quartz. Plagioclase is common in phenocrysts, but it can also occur in holocrystalline rocks in the microcrystalline matrix along with the pyroxenes and an iron–titanium oxide phase. In addition to the differences in iron content between the tholeiitic and calc-alkalic series, the latter has a higher alumina content (16 to 20 percent), and the range in silica content is larger (48 to 75 percent compared to 45 to 63 percent for the former). Hornblende and biotite phenocrysts are common in the calc-alkalic andesites and dacites but are lacking in the tholeiites except as alteration products. The dacites and rhyolites commonly have phenocrysts of plagioclase, alkali feldspar (usually sanidine), and quartz in a glassy matrix. Hornblende and plagioclase phenocrysts are more widespread in dacites than in rhyolites, which have more biotite and alkali feldspar. When occurring near volcanic vents, (openings from which volcanic materials are brought to the Earth's surface), basalts and andesites of both series are found as tuffs or agglomerates; otherwise, they typically occur as flows. Dacite and rhyolite occur as flows near vents but are most commonly found as tuffs composed of fragmented pieces of glass, phenocrysts, and rock.
The alkaline rocks typically are chemically undersaturated with respect to silica; hence they lack normative orthopyroxene (i.e., they have only one pyroxene, the calcium-rich augite) and quartz but have normative nepheline. Microscopic examination of the alkali olivine basalts usually reveals phenocrysts with an abundance of olivine, one pyroxene (augite, which is usually titanium-rich), and plagioclase. Nepheline may be seen in the matrix. Trachytes typically are leucocratic with an abundance of feldspars aligned roughly parallel to the direction of the lava flow.
Origin and distribution
Origin of magmas (magma)
Basaltic magmas that form the oceanic crust of the Earth are generated in the asthenosphere at a depth of about 70 kilometres. The mantle rocks located at depths from about 70 to 200 kilometres are believed to exist at temperatures slightly above their melting point, and possibly 1 or 2 percent of the rocks occur in the molten state. As a result, the asthenosphere behaves plastically, and upon penetrating this zone seismic waves experience a slight drop in velocity; this shell came to be known as the low velocity zone. Only after the acceptance of the plate tectonic theory has this zone become known as the asthenosphere (see plate tectonics). The most common mantle rock within the asthenosphere is peridotite, which is composed predominantly of magnesium-rich olivine, along with lesser amounts of chromium diopside and enstatite and an even smaller quantity of garnet. Peridotite may undergo partial melting to produce magmas with different compositions.
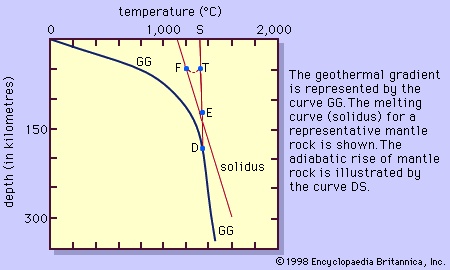 Theories on the generation of basaltic magma mainly attribute its origin to the derivation of heat from within peridotite rather than by some outside source such as the radioactive decay of uranium, thorium, and potassium, which are only of minor consequence. Because of the difference in composition between basalt and peridotite, only a small amount of heat is needed to produce about 3 to at most 25 percent melt. Many theories have been proposed, but only the simplest and most popular is discussed here. The change in the temperature of the Earth as a function of depth, given by the estimated geothermal gradient, and the experimentally based melting curve (solidus) of the peridotite are illustrated in Figure 2-->
Theories on the generation of basaltic magma mainly attribute its origin to the derivation of heat from within peridotite rather than by some outside source such as the radioactive decay of uranium, thorium, and potassium, which are only of minor consequence. Because of the difference in composition between basalt and peridotite, only a small amount of heat is needed to produce about 3 to at most 25 percent melt. Many theories have been proposed, but only the simplest and most popular is discussed here. The change in the temperature of the Earth as a function of depth, given by the estimated geothermal gradient, and the experimentally based melting curve (solidus) of the peridotite are illustrated in Figure 2--> . At depth D, the geothermal gradient curve and the solidus of the peridotite have their closest approach, but the peridotite is still solid. Diverse mechanisms have been proposed to explain the cause for the intersection here of the two curves. One theory suggests that a decrease in pressure (equivalent to depth) at constant composition and without loss of heat will cause the peridotite to melt along the curve DS. This is identical to an adiabatic cooling process (one without an overall loss or gain of heat) in which temperature will drop slightly owing to the expansion of the rock that occurs in response to the pressure decrease. The drop in temperature is about 10 times smaller than the drop in temperature along the solidus for the same decrease in pressure. Physically, the peridotite rises to a lesser depth owing to convection in the mantle (the zone below the Earth's crust) without any exchange of heat. Melting is initiated when the curve DS intersects the melting curve at point E. As the peridotite continues to rise, it will follow the melting curve, continually producing more melt. This results from the peridotite providing its own heat. To illustrate this, consider the peridotite following the adiabatic curve DS from E to point T where it is (T - F) degrees above the melting curve. Allowing the peridotite to cool at this pressure from T to F releases heat that will be consumed in the melting process. The peridotite can be thought of as making similar but infinitesimally small steps like E to T to F as it moves along the solidus. In this way heat is provided for the melting as the peridotite moves continuously along the solidus.
. At depth D, the geothermal gradient curve and the solidus of the peridotite have their closest approach, but the peridotite is still solid. Diverse mechanisms have been proposed to explain the cause for the intersection here of the two curves. One theory suggests that a decrease in pressure (equivalent to depth) at constant composition and without loss of heat will cause the peridotite to melt along the curve DS. This is identical to an adiabatic cooling process (one without an overall loss or gain of heat) in which temperature will drop slightly owing to the expansion of the rock that occurs in response to the pressure decrease. The drop in temperature is about 10 times smaller than the drop in temperature along the solidus for the same decrease in pressure. Physically, the peridotite rises to a lesser depth owing to convection in the mantle (the zone below the Earth's crust) without any exchange of heat. Melting is initiated when the curve DS intersects the melting curve at point E. As the peridotite continues to rise, it will follow the melting curve, continually producing more melt. This results from the peridotite providing its own heat. To illustrate this, consider the peridotite following the adiabatic curve DS from E to point T where it is (T - F) degrees above the melting curve. Allowing the peridotite to cool at this pressure from T to F releases heat that will be consumed in the melting process. The peridotite can be thought of as making similar but infinitesimally small steps like E to T to F as it moves along the solidus. In this way heat is provided for the melting as the peridotite moves continuously along the solidus.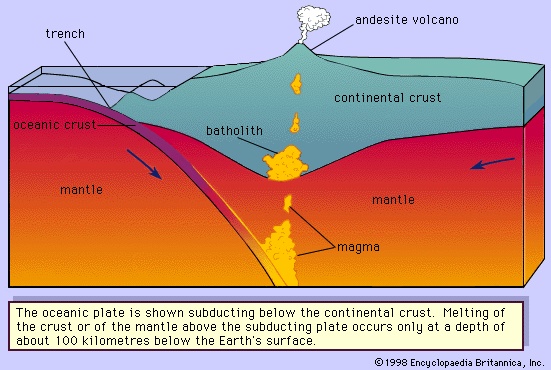 Granitic, or rhyolitic, magmas and andesitic magmas are generated at convergent plate boundaries where the oceanic lithosphere (the outer layer of the Earth composed of the crust and upper mantle) is subducted so that its edge is positioned below the edge of the continental plate or another oceanic plate. Heat will be added to the subducting lithosphere as it moves slowly into the hotter depths of the mantle. The andesitic magma is believed to be generated in the wedge of mantle rock below the crust and above the subducted plate (Figure 3-->
Granitic, or rhyolitic, magmas and andesitic magmas are generated at convergent plate boundaries where the oceanic lithosphere (the outer layer of the Earth composed of the crust and upper mantle) is subducted so that its edge is positioned below the edge of the continental plate or another oceanic plate. Heat will be added to the subducting lithosphere as it moves slowly into the hotter depths of the mantle. The andesitic magma is believed to be generated in the wedge of mantle rock below the crust and above the subducted plate (Figure 3--> ) or within the subducted plate itself. The former requires the partial melting of a “wet” peridotite. Experiments conducted at pressures simulating mantle conditions have demonstrated that peridotite will produce andesitic melts during partial melting under hydrous conditions. The latter theory suggests that the subducted basaltic crust is partially melted and may be combined with some subducted oceanic sediments to form andesites. A third theory involves the mixing of basaltic magma that was generated in the mantle with granitic or rhyolitic magma or with crustal rocks. The silicic magmas can be formed by a combination of two processes; the presence of water under pressure lowers the melting temperature by as much as 200° C (392° F) and thereby expedites magma generation. At a convergent plate boundary, the lower continental crust is heated to a temperature near its melting point by being pushed downward into hotter regions of the mantle. Basaltic or andesitic magma generated below the crust may accumulate near the Moho, which is a discontinuity that separates the Earth's crust from its mantle. As the magma cools, it crystallizes and releases its latent heat of crystallization. This evolved heat is transferred to the lower crustal rocks along with the simple heat released by cooling. If the lower crustal rocks contain some water, their melting temperatures would be lowered and the heating provided by the above processes would possibly be sufficient to partially melt the crustal rocks producing rhyolitic magma.
) or within the subducted plate itself. The former requires the partial melting of a “wet” peridotite. Experiments conducted at pressures simulating mantle conditions have demonstrated that peridotite will produce andesitic melts during partial melting under hydrous conditions. The latter theory suggests that the subducted basaltic crust is partially melted and may be combined with some subducted oceanic sediments to form andesites. A third theory involves the mixing of basaltic magma that was generated in the mantle with granitic or rhyolitic magma or with crustal rocks. The silicic magmas can be formed by a combination of two processes; the presence of water under pressure lowers the melting temperature by as much as 200° C (392° F) and thereby expedites magma generation. At a convergent plate boundary, the lower continental crust is heated to a temperature near its melting point by being pushed downward into hotter regions of the mantle. Basaltic or andesitic magma generated below the crust may accumulate near the Moho, which is a discontinuity that separates the Earth's crust from its mantle. As the magma cools, it crystallizes and releases its latent heat of crystallization. This evolved heat is transferred to the lower crustal rocks along with the simple heat released by cooling. If the lower crustal rocks contain some water, their melting temperatures would be lowered and the heating provided by the above processes would possibly be sufficient to partially melt the crustal rocks producing rhyolitic magma.Nature of magmas
Magmas are chemically complex fluid systems that differ in many ways from ordinary solutions, in which water is the solvent and the dominant constituent. They can be thought of as mutual solutions, or melts, of rock-forming components that are variously present as simple ions (ion), as complex ions and ionic groups, and as molecules. The most abundant of the simple ions in common magmas are such singly and doubly charged cations (cation) as Na+, K+, Ca2+, Mg2+, and Fe2+. Because these ions can move about rather freely in the system, they occupy no fixed positions with respect to other ions that are present. In contrast, the smaller and more highly charged cations, notably Si4+, Al3+, and (to a lesser degree) Fe3+, are surrounded or screened by O2− ions and other anions (negative ions) to form parts of relatively stable complex ions such as (SiO4)4−, (AlO4)5−, and (FeO6)9−. Simple anions (anion), including F−, Cl−, O2−, and (OH)−, ordinarily are present in much smaller amounts. Water, hydrochloric acid (HCl), hydrogen fluoride (HF), carbon dioxide (CO2), and other volatile molecular substances occur as well, generally in equilibrium with ionic forms such as (OH)−, Cl−, F−, and (CO3)2−.
Because the bond that unites silicon and oxygen is a remarkably strong one, (SiO4)4− ions are stable in magmas even at exceedingly high temperatures. They also tend to join with one another, or polymerize (polymerization), to form more complex anionic groups, a tendency that is especially great in the more silicic magmas. The joining is accomplished by a sharing of oxygen ions between adjacent silicon ions to form Si-O-Si bridges like those in many silicate and aluminosilicate minerals; in the simplest such case, (Si2O7)6− ions are the result. Because the (AlO4)5− ions also have a strong tendency to polymerize, most of the large ionic groups in magmas probably contain both silicon and aluminum ions. These groups, which resemble the frameworks of many rock-forming minerals but are geometrically less regular, significantly affect the viscosity and crystallization of magmas.
The viscosity of magmas, which spans an enormous range of values, affects their flow behaviour, the movements of crystals and inclusions of foreign matter within them, the diffusion of materials through them, the growth of crystals from them, and the explosivity of eruptions (when aided by growth of gas bubbles near the surface). Lava flows are thin and rapid for low-viscosity magmas, but thick and slow for viscous flows. Fluid magma promotes the growth of large crystals such as the ones found in pegmatites, but crystal growth is prevented in viscous magmas, which usually are quenched as glass. Highly explosive eruptions such as occurred at Mount St. Helens commonly result from gas bubbles nucleating, growing, and rising in a highly viscous magma. It can be demonstrated thermodynamically that the overpressure (excess rock pressure) developed in growing and rising bubbles is inversely proportional to their radii. In fluid magmas, gas bubbles grow large in size and rise quickly, which causes their pressures to be expended; hence, only a spectacular fountaining of hot lava is observed at the surface. In contrast, a viscous magma prevents the growth of bubbles, so that they will rise slowly while retaining excess pressure; as a result, the associated volcano erupts violently. Energies equivalent to the amount produced by several nuclear bombs are released in such explosions. Viscosity increases greatly with decreasing temperature and less markedly with increasing pressure. It also can be governed in part by the amount and distribution of any solid materials or bubbles of gas present, which both tend to increase viscosity. Finally, it varies considerably among magmas of differing gross composition, mainly because of the differences in the degree of Si-O and Al-O polymerization. Thus, highly silicic magmas generally are more viscous than mafic ones by several orders of magnitude, a difference reflected by contrasts in the eruptive behaviour of rhyolitic and basaltic lavas. Basaltic magmas at 1,100° C can be at least 100,000 times more viscous than water at room temperature, whereas rhyolitic magmas at 800° C are at least 10 million times more viscous than room-temperature water. The presence of volatile constituents can markedly increase the fluidity of magmas, even those that are rich in SiO2. This effect has been attributed to the breaking of Si-O-Si bridges through substitution of ions such as F− and (OH)− for shared O2− ions in elements of the polymerized groups.
A typical magma can be broadly viewed as an assemblage of relatively large and rather closely packed oxygen ions, among which some cations have considerable mobility; others, such as Si4+ and Al3+, tend to occupy positions that are more fixed. The entire system is a dynamic one, however, and even the largest of the Si-O and Al-O ion groups are constantly changing form and position as bonds are broken and new ones are established. If the magma quickly loses thermal energy and cools to a glass, these internal movements are sharply restricted, and the various constituents become essentially frozen in position. If cooling is slower, the contained complex ions and polymerized ion groups have time to assume more regular arrangements and to be stabilized by cations of appropriate size, charge, and other properties. Crystalline solids are thereby formed. Their regular internal structure is relatively conserving of space, and so they have somewhat higher specific gravities than the magma from which they were nourished.
Crystallization from magmas
The forsterite-cristobalite system
Because magmas are multicomponent solutions, they do not crystallize at a single temperature at a given pressure like water at 0° C and one atmosphere pressure. Rather, they crystallize over a wide range of temperatures beginning at liquidus temperatures for basaltic magmas as high as 1,150° C and ending as a complete solid at a low solidus temperature of about 800° C. During their crystallization at constant pressure, common minerals that make up basaltic magma (e.g., olivine) become unstable at some temperature and react with the liquid to form a more stable phase. In the case of olivine, this phase is pyroxene. This reaction relationship is best illustrated with the use of a phase diagram of a portion of the olivine Mg2SiO4 (forsterite) + SiO2 (cristobalite, a high-temperature form of quartz) binary system at one atmosphere.
Consider a mixture X of two minerals in the proportions 28 percent cristobalite and 72 percent forsterite. At a temperature of 1,601° C, this mixture is entirely liquid. At temperatures below 1,557° C, forsterite (Fo) and enstatite (En) are stable, but between 1,557° and 1,600° C, forsterite and the liquid whose composition is represented by L are in equilibrium. At a temperature of 1,570° C, there is about 7 percent forsterite and 93 percent liquid. As the liquid X cools, it intersects the liquidus freezing curve at a temperature of 1,600° C, where forsterite begins to crystallize. As the temperature drops further, the liquid follows the liquidus down toward R, the peritectic point (incongruent melting point in a binary system), while it continually crystallizes more forsterite. It should be noted that the liquid composition is becoming enriched in silica, until at R, it has more silica than enstatite. At this point the forsterite reacts with the liquid to yield two moles of MgSiO3 (enstatite) for every mole of Mg2SiO4 that combines with one mole of SiO2 removed from the liquid R. This can be written as a chemical equation: Mg2SiO4 + SiO2 ⇄ 2MgSiO3. Because SiO2 is removed from the liquid R, a proportionate amount of enstatite must be crystallized from the liquid to keep its composition at point R. In the case of the starting composition X, which is depleted in SiO2 relative to enstatite, the peritectic liquid, R, will be consumed by the reaction prior to the forsterite, and the resultant mixture will consist of forsterite and enstatite. However, in the case in which the starting composition is Y, which is enriched in silica relative to enstatite, the forsterite will be depleted before the liquid, and the reaction will yield the liquid and enstatite. Only in the case where the starting composition matches that of enstatite will the liquid and the forsterite be consumed at the same time, leaving only enstatite. The starting composition X represents the most common crystallization behaviour for saturated tholeiitic basaltic magmas; consequently, these magmas will experience a reaction between the liquid and the olivine, forsterite, at some point during their crystallization. This means that the liquid will be consumed by the reaction with forsterite and crystallization will cease. If, however, forsterite can be removed physically from the liquid before the reaction can occur, the reaction will be prevented and the peritectic liquid will remain to crystallize the pyroxene, enstatite, and move down toward the eutectic temperature where cristobalite and enstatite will crystallize.
The albite-anorthite system
 Most of the common minerals found in igneous rocks are solid-solution (solid solution) phases. These include olivine, pyroxene, amphibole, biotite, and plagioclase feldspars. Crystallization behaviour is illustrated best by using the NaAlSi3O8 (albite or Ab)–CaAl2Si2O8 (anorthite or An) plagioclase system shown in Figure 4-->
Most of the common minerals found in igneous rocks are solid-solution (solid solution) phases. These include olivine, pyroxene, amphibole, biotite, and plagioclase feldspars. Crystallization behaviour is illustrated best by using the NaAlSi3O8 (albite or Ab)–CaAl2Si2O8 (anorthite or An) plagioclase system shown in Figure 4--> . Consider a liquid of composition L (60 percent An + 40 percent Ab) which is at an initial temperature of 1,500° C. On cooling it will begin crystallizing plagioclase with 85 percent An (point P on the solidus) at the liquidus temperature of about 1,470° C. As cooling continues further, the liquid will move down the liquidus toward B while simultaneously reacting continuously with the early-formed plagioclase to convert it to a homogeneous plagioclase that is more albitic and in equilibrium with the liquid. For example, when the liquid has reached A, at 1,400° C, about 65 percent plagioclase with about 73 percent An (point O on the solidus) has crystallized from the liquid, which is now at about 36 percent An and 64 percent Ab. Finally, when the temperature of about 1,330° C is reached (B in the figure), the last small amount of the liquid of composition 20 percent An + 80 percent Ab is consumed in the reaction and a homogeneous plagioclase of 60 percent An + 40 percent Ab remains (point S). Now consider the case in which the liquid is prevented from reacting with the early-formed plagioclase. This may be achieved by physically removing the plagioclase immediately after its formation or by cooling the liquid faster than the reaction process can consume the plagioclase. The liquid could theoretically reach the pure Ab composition at 1,100° C, where it will disappear into the crystallizing albite. A whole range of plagioclase compositions from An84 to An00 will be preserved in the cooling process.
. Consider a liquid of composition L (60 percent An + 40 percent Ab) which is at an initial temperature of 1,500° C. On cooling it will begin crystallizing plagioclase with 85 percent An (point P on the solidus) at the liquidus temperature of about 1,470° C. As cooling continues further, the liquid will move down the liquidus toward B while simultaneously reacting continuously with the early-formed plagioclase to convert it to a homogeneous plagioclase that is more albitic and in equilibrium with the liquid. For example, when the liquid has reached A, at 1,400° C, about 65 percent plagioclase with about 73 percent An (point O on the solidus) has crystallized from the liquid, which is now at about 36 percent An and 64 percent Ab. Finally, when the temperature of about 1,330° C is reached (B in the figure), the last small amount of the liquid of composition 20 percent An + 80 percent Ab is consumed in the reaction and a homogeneous plagioclase of 60 percent An + 40 percent Ab remains (point S). Now consider the case in which the liquid is prevented from reacting with the early-formed plagioclase. This may be achieved by physically removing the plagioclase immediately after its formation or by cooling the liquid faster than the reaction process can consume the plagioclase. The liquid could theoretically reach the pure Ab composition at 1,100° C, where it will disappear into the crystallizing albite. A whole range of plagioclase compositions from An84 to An00 will be preserved in the cooling process.Bowen's reaction series
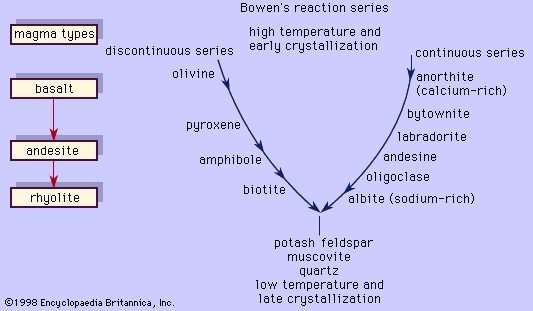 These two examples illustrate two principal reactions that occur during crystallization of common magmas, one discontinuous (the olivine-liquid-pyroxene reaction) and the other continuous (the plagioclase-liquid reaction). This was recognized first by the American petrologist Norman L. Bowen, who arranged the reactions in the form shown in Figure 5-->
These two examples illustrate two principal reactions that occur during crystallization of common magmas, one discontinuous (the olivine-liquid-pyroxene reaction) and the other continuous (the plagioclase-liquid reaction). This was recognized first by the American petrologist Norman L. Bowen, who arranged the reactions in the form shown in Figure 5--> ; in his honour, the mineral series has since been called the Bowen's reaction series. The left branch of the Y-shaped arrangement consists of the discontinuous series that begins with olivine at the highest temperature and progresses through pyroxene, amphibole, and biotite as the temperature decreases. This series is discontinuous because the reaction occurs at a fixed temperature at constant pressure wherein the early-formed mineral is converted to a more stable crystal. Each mineral in the series displays a different silicate structure that exhibits increased polymerization as the temperature drops; olivine belongs to the island silicate structure type; pyroxene, the chain; amphibole, the double chain; and biotite, the sheet. On the other hand, the right branch is the continuous reaction series in which plagioclase is continuously reacting with the liquid to form a more albitic phase as the temperature decreases. In both cases, the liquid is consumed in the reaction. When the two reaction series converge at a low temperature, minerals that will not react with the remaining liquid approach eutectic crystallization. Potash feldspar, muscovite, and quartz are crystallized. The phases that are crystallized first are the common minerals that compose basalt or gabbro, like bytownite or labradorite with pyroxene and minor amounts of olivine. Andesite or diorite minerals, such as andesine with either pyroxene or amphibole, crystallize next and are followed by orthoclase and quartz, which are the essential constituents of rhyolite or granite. A basaltic liquid at the top of the Y can descend to the bottom of the series to crystallize quartz only if the earlier reactions are prevented. As demonstrated above, complete reactions between early-formed minerals and the liquid depletes the supply of the liquid, thereby curtailing the progression down the series. One means by which basaltic magma can be transformed to rocks lower in the series is by fractional crystallization. In this process, the early-formed minerals are removed from the liquid by gravity (such minerals as olivine and pyroxene are denser than the liquid from which they crystallized), and so unreacted liquid remains later in the series.
; in his honour, the mineral series has since been called the Bowen's reaction series. The left branch of the Y-shaped arrangement consists of the discontinuous series that begins with olivine at the highest temperature and progresses through pyroxene, amphibole, and biotite as the temperature decreases. This series is discontinuous because the reaction occurs at a fixed temperature at constant pressure wherein the early-formed mineral is converted to a more stable crystal. Each mineral in the series displays a different silicate structure that exhibits increased polymerization as the temperature drops; olivine belongs to the island silicate structure type; pyroxene, the chain; amphibole, the double chain; and biotite, the sheet. On the other hand, the right branch is the continuous reaction series in which plagioclase is continuously reacting with the liquid to form a more albitic phase as the temperature decreases. In both cases, the liquid is consumed in the reaction. When the two reaction series converge at a low temperature, minerals that will not react with the remaining liquid approach eutectic crystallization. Potash feldspar, muscovite, and quartz are crystallized. The phases that are crystallized first are the common minerals that compose basalt or gabbro, like bytownite or labradorite with pyroxene and minor amounts of olivine. Andesite or diorite minerals, such as andesine with either pyroxene or amphibole, crystallize next and are followed by orthoclase and quartz, which are the essential constituents of rhyolite or granite. A basaltic liquid at the top of the Y can descend to the bottom of the series to crystallize quartz only if the earlier reactions are prevented. As demonstrated above, complete reactions between early-formed minerals and the liquid depletes the supply of the liquid, thereby curtailing the progression down the series. One means by which basaltic magma can be transformed to rocks lower in the series is by fractional crystallization. In this process, the early-formed minerals are removed from the liquid by gravity (such minerals as olivine and pyroxene are denser than the liquid from which they crystallized), and so unreacted liquid remains later in the series.Assimilation
Another method of creating different daughter magmas from a parent is by having the latter react with its wall rocks. Consider a magma that is crystallizing pyroxene and labradorite. If the magma tears from its wall minerals, say, olivine and anorthite, which are formed earlier than pyroxene and labradorite in the series, they will react with the liquid to form these same minerals with which the magma is in equilibrium. The heat for driving this reaction comes directly from the magma itself. More pyroxene and labradorite will crystallize during the reaction and will release their latent heats of crystallization. On the other hand, if a mineral (quartz, for example) formed at a later stage than pyroxene or labradorite falls from the rock wall into the magma, the latent heat provided by further crystallization of pyroxene and labradorite will cause it to dissolve. This situation will occur only if the quartz from the wall rock is at a lower temperature than the magma. It will cause the magma to transfer its heat to the quartz in a cooling process. The cooling of the magma will necessarily be accompanied by the crystallization of the minerals already present. In both cases, the composition of the parent magma will be changed by the xenolithic (foreign rock) contamination. The contaminant need not belong to the reaction series in order for it to cause reactions or dissolution. In most cases, the end result will be a shift from the original composition of the parent magma toward that of the contaminant. This process in which wall rocks are incorporated into the magma is called assimilation. Because assimilation is accompanied by crystallization, it is likely that both fractional crystallization and assimilation will take place simultaneously. This combined process, referred to as AFC for assimilation–fractional crystallization, has been proposed as the mechanism by which andesites are produced from basalts.
Volatile constituents and late magmatic processes
Effects of water and other volatiles
Water and most other volatile substances profoundly influence the properties and behaviour of magmas in which they are dissolved. They reduce viscosity, lower temperatures of crystallization by tens to hundreds of degrees, and participate directly in the formation of minerals that contain essential hydroxyl (OH) or elements such as the halogens. They also increase rates of crystallization and reaction, especially when they are present as a fluid phase distinct from the magma. In general, however, they have only a limited influence on the sequence of magmatic crystallization, except in the latest stages of the reaction series.
The relatively low confining pressures in volcanic environments permit ready escape of volatile constituents, which nonetheless leave their imprint in the form of special mineral assemblages and a variety of textural and structural features among the volcanic rocks. Under the higher pressures of plutonic environments, these constituents tend to be maintained in magmatic solution and to be increasingly concentrated as crystallization progresses with falling temperature. Few members of the reaction series require them as compositional contributors; water, for example, is not thus used until amphiboles or micas begin to form, and even then the amounts removed from the melt rarely are large. Escape of volatiles from the system can occur “osmotically” if the enclosing rocks are pervious to them but not to the magma, but in general they are fractionated in favour of the residual melt until their concentration reaches the limit of solubility under the prevailing conditions of temperature and effective confining pressure. When this happens, normally at a very late stage of magmatic crystallization, they are exsolved from the melt as a separate fluid phase that under most circumstances is a supercritical gas. This process has been referred to as resurgent boiling, a somewhat misleading term because the exsolved fluid is not necessarily expelled from the system.
Pegmatites (pegmatite) and late-stage mineralization
Coexistence of residual magma and a volatile-rich fluid (generally aqueous) promotes the partitioning and segregation of constituents, as well as the growth of very large crystals. The exsolved fluid, with its very low viscosity, not only can move readily through open spaces in the nearly solid igneous rock and in adjacent rocks but also serves as a medium through which various substances can diffuse rapidly in response to concentration gradients. Thus, it plays an important role in the formation of such special rock types as the pegmatites and lamprophyres, special features such as miaroles and plumose mineral aggregates, and many kinds of ore deposits whose constituents are derived from the original magma.
Most plutonic systems remain at elevated temperatures for long periods of time after all magma has been used up, and during these periods hydrothermal conditions normally obtain. These depend upon the continued presence of a typically aqueous fluid that further facilitates crystallization and exchanges of materials. It speeds up exsolution within homogeneous solid phases and devitrification of any glass that may be present, and it is a potent agent in the alteration, leaching, and replacement of minerals. Rock textures thereby are modified, especially along boundaries between original mineral grains, and details of composition also can be much changed. In some instances the bulk chemistry of the rock is markedly affected.
The hydrothermal alterations favour development of phases such as albite, carbonates, chlorites, clay minerals, epidotes, iron oxides, micas, silica minerals, talc, and zeolites, and many of them are accompanied by gross changes in volume.
Forms of occurrence
Extrusive igneous rocks
Extrusive igneous rocks are the products of volcanic activity. They appear at the surface as molten lava that spreads in sheets and hardens, or they are made up of fragments of magma ejected from vents by violent gaseous explosions. Large-scale extrusive features include stratovolcanoes (composite cones), shield volcanoes, lava domes, and cinder cones. Smaller extrusive features include lava flows known as pahoehoe and aa. For a detailed description of the principal forms of extrusive igneous rocks, see volcano: Volcanic landforms: Major types of volcanic landforms (volcano).
Intrusive igneous rocks
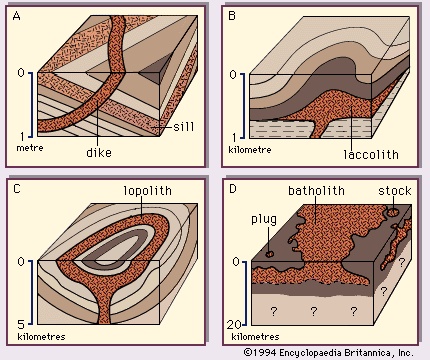 Erosion of volcanoes will immediately expose shallow intrusive bodies such as volcanic necks and diatremes (see Figure 6-->
Erosion of volcanoes will immediately expose shallow intrusive bodies such as volcanic necks and diatremes (see Figure 6--> ). A volcanic neck is the “throat” of a volcano and consists of a pipelike conduit filled with hypabyssal rocks. Ship Rock in New Mexico and Devil's Tower in Wyoming are remnants of volcanic necks, which were exposed after the surrounding sedimentary rocks were eroded away. Many craterlike depressions may be filled with angular fragments of country rock (breccia) and juvenile pyroclastic debris. When eroded, such a depression exposes a vertical funnel-shaped pipe that resembles a volcanic neck with the exception of the brecciated filling. These pipes are dubbed diatremes. Many diatremes are formed by explosion resulting from the rapid expansion of gas—carbon dioxide and water vapour. These gases are released by the rising magma owing to the decrease in pressure as it nears the surface. Some diatremes contain kimberlite, a peridotite that contains a hydrous mineral called phlogopite. Kimberlite may contain diamonds.
). A volcanic neck is the “throat” of a volcano and consists of a pipelike conduit filled with hypabyssal rocks. Ship Rock in New Mexico and Devil's Tower in Wyoming are remnants of volcanic necks, which were exposed after the surrounding sedimentary rocks were eroded away. Many craterlike depressions may be filled with angular fragments of country rock (breccia) and juvenile pyroclastic debris. When eroded, such a depression exposes a vertical funnel-shaped pipe that resembles a volcanic neck with the exception of the brecciated filling. These pipes are dubbed diatremes. Many diatremes are formed by explosion resulting from the rapid expansion of gas—carbon dioxide and water vapour. These gases are released by the rising magma owing to the decrease in pressure as it nears the surface. Some diatremes contain kimberlite, a peridotite that contains a hydrous mineral called phlogopite. Kimberlite may contain diamonds.


 Dikes (dike) are usually tabular bodies that may radiate from the central vent of a volcano or from a volcanic neck (see Figure 6-->
Dikes (dike) are usually tabular bodies that may radiate from the central vent of a volcano or from a volcanic neck (see Figure 6--> ). Not all dikes are associated with volcanoes, but they can be distinguished by their discordant relationship with the structure of the country rock that they cut across. Many dikes are only a few metres wide, but large ones, such as the dike that feeds the Muskox intrusion in the Northwest Territories of Canada, reach widths of more than 150 metres. Related to dikes are features that maintain a concordant relationship with the structure of the country rocks. Magmas may force their way between layers of country rock and solidify parallel to them to form sills (sill) (see Figure 6-->
). Not all dikes are associated with volcanoes, but they can be distinguished by their discordant relationship with the structure of the country rock that they cut across. Many dikes are only a few metres wide, but large ones, such as the dike that feeds the Muskox intrusion in the Northwest Territories of Canada, reach widths of more than 150 metres. Related to dikes are features that maintain a concordant relationship with the structure of the country rocks. Magmas may force their way between layers of country rock and solidify parallel to them to form sills (sill) (see Figure 6--> ). On the west bank of the Hudson River opposite New York City, the 300-metre-thick Palisades sill is exposed and can be traced for 80 kilometres. A laccolith also is concordant with country rock, but it is distinguished from a sill by having a flat floor with a domed (mushroom-shaped) roof (see Figure 6-->
). On the west bank of the Hudson River opposite New York City, the 300-metre-thick Palisades sill is exposed and can be traced for 80 kilometres. A laccolith also is concordant with country rock, but it is distinguished from a sill by having a flat floor with a domed (mushroom-shaped) roof (see Figure 6--> ). Laccoliths were first described in the Henry Mountains of Utah, where they may measure up to 200 metres thick with basal diameters exceeding three kilometres. Rocks of intermediate silica content generally make up these domed intrusions. In contrast, lopoliths (lopolith) are saucer-shaped bodies with a concave upward roof and floor and are commonly composed of mafic rocks. Lopoliths are huge in size; the Bushveld intrusive complex in South Africa, for example, has an area of about 66,000 square kilometres and an exposed thickness of 8 kilometres. The Muskox intrusion, mentioned above, is another large lopolith, which is estimated to be about 80 kilometres long and 11 kilometres wide (roof rocks covering part of the intrusion prevent an exact measurement). These lopoliths are commonly layered with igneous minerals and rocks; in the Bushveld intrusion, one layer about 1 metre thick consisting of almost pure chromite (an ore of chromium) extends for tens of kilometres. Large irregularly shaped plutons (pluton) are called either stocks or batholiths (batholith) (see Figure 6-->
). Laccoliths were first described in the Henry Mountains of Utah, where they may measure up to 200 metres thick with basal diameters exceeding three kilometres. Rocks of intermediate silica content generally make up these domed intrusions. In contrast, lopoliths (lopolith) are saucer-shaped bodies with a concave upward roof and floor and are commonly composed of mafic rocks. Lopoliths are huge in size; the Bushveld intrusive complex in South Africa, for example, has an area of about 66,000 square kilometres and an exposed thickness of 8 kilometres. The Muskox intrusion, mentioned above, is another large lopolith, which is estimated to be about 80 kilometres long and 11 kilometres wide (roof rocks covering part of the intrusion prevent an exact measurement). These lopoliths are commonly layered with igneous minerals and rocks; in the Bushveld intrusion, one layer about 1 metre thick consisting of almost pure chromite (an ore of chromium) extends for tens of kilometres. Large irregularly shaped plutons (pluton) are called either stocks or batholiths (batholith) (see Figure 6--> ), depending on their sizes. Plutons larger than 100 square kilometres in area are termed batholiths, while those of lesser size are called stocks. It may be possible, however, that some stocks are the visible portions of batholiths that have not been exposed by erosion. Batholiths (from the Greek word bathos, meaning depth) are deep-seated crustal intrusions, whereas stocks may be formed at shallow depths only a few kilometres below the surface. Rocks ranging from quartz diorite to granite are commonly found in batholiths. Large batholiths in North America include the Sierra Nevada, the Idaho, and the Coast Range, which is about 600 kilometres long and 200 kilometres wide and extends from the Alaskan border through British Columbia to Washington state. Many pulses of intrusions contribute to the formation of these large bodies; for example, eight episodes of activity have been recognized in the Sierra Nevada batholith. They are formed, therefore, by the coalescence of many smaller batholiths and stocks.
), depending on their sizes. Plutons larger than 100 square kilometres in area are termed batholiths, while those of lesser size are called stocks. It may be possible, however, that some stocks are the visible portions of batholiths that have not been exposed by erosion. Batholiths (from the Greek word bathos, meaning depth) are deep-seated crustal intrusions, whereas stocks may be formed at shallow depths only a few kilometres below the surface. Rocks ranging from quartz diorite to granite are commonly found in batholiths. Large batholiths in North America include the Sierra Nevada, the Idaho, and the Coast Range, which is about 600 kilometres long and 200 kilometres wide and extends from the Alaskan border through British Columbia to Washington state. Many pulses of intrusions contribute to the formation of these large bodies; for example, eight episodes of activity have been recognized in the Sierra Nevada batholith. They are formed, therefore, by the coalescence of many smaller batholiths and stocks.Distribution of igneous rocks on the Earth's surface
Divergent plate boundaries
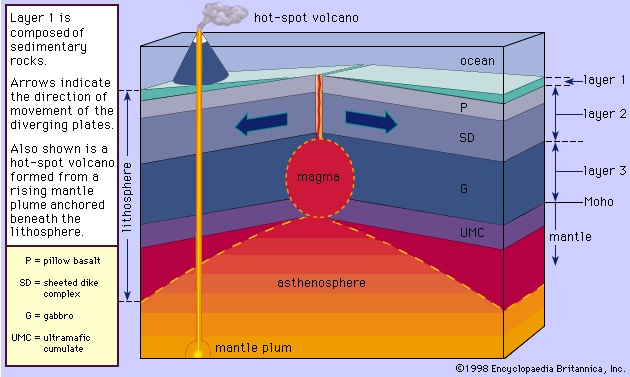 Most of the igneous activity on the Earth is restricted to a narrow zone that is related intimately with the motions of the lithospheric plates. Indeed, the composition of the magma, the types of volcanism, and the characteristics of intrusions are governed to a large extent by plate tectonics. The magmatism at divergent plate boundaries along the crests of the oceanic rises and ridges is mostly unseen except in places where the volcanic activity occurs subaerially (e.g., Iceland, which sits on the Mid-Atlantic Ridge). Along these divergent boundaries, the erupted basalts have such a restricted compositional range that they are referred to as mid-ocean-ridge basalt (MORB). They are subalkaline tholeiites that contain olivine in the norm and less than 0.25 percent potash. The chemistry suggests that MORB was generated from a mantle that was depleted of volatile elements (e.g., lanthanum 【La】, cerium 【Ce】, sodium, and potassium) in a previous partial melting process. A wide rift valley marks the crest of most of the oceanic ridges and rises. The valley is bounded by faults created by the divergent forces and is floored in its centre by a fracture zone (a mass of rock with many small breakages). These faults and fractures are the conduits for the MORB magmas that flood the valley, build volcanoes, and produce dikes by filling the conduits. Layer 2 of the oceanic crust results from these magmatic activities (see Figure 7-->
Most of the igneous activity on the Earth is restricted to a narrow zone that is related intimately with the motions of the lithospheric plates. Indeed, the composition of the magma, the types of volcanism, and the characteristics of intrusions are governed to a large extent by plate tectonics. The magmatism at divergent plate boundaries along the crests of the oceanic rises and ridges is mostly unseen except in places where the volcanic activity occurs subaerially (e.g., Iceland, which sits on the Mid-Atlantic Ridge). Along these divergent boundaries, the erupted basalts have such a restricted compositional range that they are referred to as mid-ocean-ridge basalt (MORB). They are subalkaline tholeiites that contain olivine in the norm and less than 0.25 percent potash. The chemistry suggests that MORB was generated from a mantle that was depleted of volatile elements (e.g., lanthanum 【La】, cerium 【Ce】, sodium, and potassium) in a previous partial melting process. A wide rift valley marks the crest of most of the oceanic ridges and rises. The valley is bounded by faults created by the divergent forces and is floored in its centre by a fracture zone (a mass of rock with many small breakages). These faults and fractures are the conduits for the MORB magmas that flood the valley, build volcanoes, and produce dikes by filling the conduits. Layer 2 of the oceanic crust results from these magmatic activities (see Figure 7--> ). As the plates diverge, MORB becomes the ocean floor on which oceanic sediments (layer 1) are deposited. This makes MORB the most abundant rock on the surface of the Earth.
). As the plates diverge, MORB becomes the ocean floor on which oceanic sediments (layer 1) are deposited. This makes MORB the most abundant rock on the surface of the Earth.Below the collection of lavas and dikes in layer 2 are found gabbro and diorite. They represent the plutonic rocks formed as a result of differentiation of the MORB magma that fed the volcanic activity along the rift. (Differentiation is the process in which more than one rock type is derived from a single parent magma.) These coarse-grained intrusives account for about 4 to 5 kilometres of layer 3, which rests on a sequence of layered ultramafic rocks. The rocks were formed by the gravitative accumulation of mafic minerals from the original MORB magma that filled a large chamber below the ridge axis. Below this layered sequence is mantle rock that is highly deformed and depleted (of elements such as lanthanum, cerium, sodium, and potassium that have been removed by repeated partial melting). Because seismic waves cannot distinguish between layered ultramafic rocks, which are not true mantle rocks, and ultramafic mantle rocks, the Moho actually is positioned between layer 3 and the layered ultramafics. The sequences consisting of layer 1 (limestone and chert sedimentary rocks), layer 2 of MORB lavas and dikes, and layer 3 of gabbro and diorite and the ultramafic rocks are known as ophiolites. Many geologists believe that ophiolites formed at oceanic ridges were emplaced by tectonic forces at convergent plate boundaries and then became exposed in highly deformed orogenic (mountain) belts. In fact, the same sequences of rocks were first reported in the Alps and were considered deep-seated intrusions. Some geologists still argue that all ophiolites were not formed at divergent plate boundaries.
 Away from the axis of divergence, the composition of the volcanic rocks becomes more diverse. Most of the magmatism is related to hot spots, which are hot rising plumes of mantle rock that are anchored beneath the moving lithospheric plates (see Figure 7-->
Away from the axis of divergence, the composition of the volcanic rocks becomes more diverse. Most of the magmatism is related to hot spots, which are hot rising plumes of mantle rock that are anchored beneath the moving lithospheric plates (see Figure 7--> ). The Hawaiian (Hawaii) Islands owe their existence to the magmatism associated with a hot spot that currently is located just southeast of the large island of Hawaii. This mantle plume not only provides magma for the eruptions at Kilauea Volcano but also is responsible for the submarine volcano named Loihi that will eventually become a new island. Most of the islands are built on a tholeiite basalt base, but the caps of the volcanoes are alkali basalts. The final episodes of volcanic activity on an island are extremely undersaturated; nephelinites and olivine melilite nephelinites are common products. The alkali basalts have differentiated to more silica-rich compositions, with hawaiites, mugearites, and trachytes being erupted in minor amounts. The two active volcanoes on Hawaii, Mauna Loa and Kilauea, are still erupting tholeiite basalts. Tholeiites on all the islands far from the ocean ridge crests are different from MORB in that they are enriched in lanthanum, cerium, sodium, and potassium. Early in the Earth's history, a high-magnesium, high-temperature mafic magma called komatiite erupted from hot spots. Since most komatiites are only found in Archean regions, they are thought to be evidence for the Earth being hotter than when it was initially formed. The youngest komatiite was recently discovered on the island of Gorgona, Colom.
). The Hawaiian (Hawaii) Islands owe their existence to the magmatism associated with a hot spot that currently is located just southeast of the large island of Hawaii. This mantle plume not only provides magma for the eruptions at Kilauea Volcano but also is responsible for the submarine volcano named Loihi that will eventually become a new island. Most of the islands are built on a tholeiite basalt base, but the caps of the volcanoes are alkali basalts. The final episodes of volcanic activity on an island are extremely undersaturated; nephelinites and olivine melilite nephelinites are common products. The alkali basalts have differentiated to more silica-rich compositions, with hawaiites, mugearites, and trachytes being erupted in minor amounts. The two active volcanoes on Hawaii, Mauna Loa and Kilauea, are still erupting tholeiite basalts. Tholeiites on all the islands far from the ocean ridge crests are different from MORB in that they are enriched in lanthanum, cerium, sodium, and potassium. Early in the Earth's history, a high-magnesium, high-temperature mafic magma called komatiite erupted from hot spots. Since most komatiites are only found in Archean regions, they are thought to be evidence for the Earth being hotter than when it was initially formed. The youngest komatiite was recently discovered on the island of Gorgona, Colom.Convergent plate boundaries
 Igneous rocks associated with convergent plate boundaries have the greatest diversity. In this case, granite batholiths underlie the great composite volcanoes and consist of rocks ranging from basalt through andesite to dacite and rhyolite. These boundaries are destructive and consume the subducting oceanic lithosphere formed at the divergent centres. The rocks generated, however, are added on (accreted) to the continent. Oceanic trenches outline the junction of the colliding plates, but the igneous activity takes place on the overriding plate along a line at least about 100 kilometres above the subducting plate (see Figure 3-->
Igneous rocks associated with convergent plate boundaries have the greatest diversity. In this case, granite batholiths underlie the great composite volcanoes and consist of rocks ranging from basalt through andesite to dacite and rhyolite. These boundaries are destructive and consume the subducting oceanic lithosphere formed at the divergent centres. The rocks generated, however, are added on (accreted) to the continent. Oceanic trenches outline the junction of the colliding plates, but the igneous activity takes place on the overriding plate along a line at least about 100 kilometres above the subducting plate (see Figure 3--> ). In other words, almost no volcanism occurs between this 100-kilometre line (called the volcanic front) and the trench. The horizontal distance between the trench and the volcanic front depends on the angle of subduction; the steeper the angle, the shorter the distance. Volcanism occurs from this volcanic axis inland for a few hundred kilometres. The dominant rock constituting the composite volcanoes is andesite, but in some younger island arcs basalt tends to be more common, and in older volcanic areas dacite or rhyolite becomes prominent. Two different series of rocks are found in some volcanic chains. In Japan a tholeiitic series and a calc-alkalic series sometimes erupt from the same volcano. The former is characterized by lower magnesium, potassium, nickel, chromium, uranium, and thorium and a higher iron:magnesium ratio. Mineralogically, the tholeiitic series characteristically contains pigeonite (a low-calcium monoclinic pyroxene) in the groundmass of the basalts and andesites. The calc-alkalic series lacks pigeonite but instead has hypersthene. Most of the composite volcanoes of the Cascades Range in Oregon and Washington in the northwestern United States are characteristically calc-alkalic. In some volcanic arcs in areas farthest from the trench, a potassic series is found. In Japan the volcanoes within the Sea of Japan and farthest from the Japan Trench have alkali basalt compositions. Recent discoveries in modern convergent margins have identified igneous rocks within the oceanic trench sediments. These occur in regions where a mid-ocean ridge is being subducted. This creates higher heat flow and different types of igneous rocks, termed trondhjemite-tonalite-dacite (TTD) suites and alkaline, mafic, and felsic types.
). In other words, almost no volcanism occurs between this 100-kilometre line (called the volcanic front) and the trench. The horizontal distance between the trench and the volcanic front depends on the angle of subduction; the steeper the angle, the shorter the distance. Volcanism occurs from this volcanic axis inland for a few hundred kilometres. The dominant rock constituting the composite volcanoes is andesite, but in some younger island arcs basalt tends to be more common, and in older volcanic areas dacite or rhyolite becomes prominent. Two different series of rocks are found in some volcanic chains. In Japan a tholeiitic series and a calc-alkalic series sometimes erupt from the same volcano. The former is characterized by lower magnesium, potassium, nickel, chromium, uranium, and thorium and a higher iron:magnesium ratio. Mineralogically, the tholeiitic series characteristically contains pigeonite (a low-calcium monoclinic pyroxene) in the groundmass of the basalts and andesites. The calc-alkalic series lacks pigeonite but instead has hypersthene. Most of the composite volcanoes of the Cascades Range in Oregon and Washington in the northwestern United States are characteristically calc-alkalic. In some volcanic arcs in areas farthest from the trench, a potassic series is found. In Japan the volcanoes within the Sea of Japan and farthest from the Japan Trench have alkali basalt compositions. Recent discoveries in modern convergent margins have identified igneous rocks within the oceanic trench sediments. These occur in regions where a mid-ocean ridge is being subducted. This creates higher heat flow and different types of igneous rocks, termed trondhjemite-tonalite-dacite (TTD) suites and alkaline, mafic, and felsic types.In older areas of convergence, the composite volcanoes have been eroded, exposing the deeper plutonic granite batholiths that extend the entire length of the convergent boundaries. The batholiths are predominantly granodiorite, but gabbro through granite occur as well. It seems anomalous to find diorite, the plutonic equivalent of andesite, in low abundance since andesite is the dominant rock type of the volcanoes that were above these batholiths. Two basic types of granite have been recognized. The more common variety is located closer to the trench, has hornblende as its mafic mineral, is enriched in sodium and calcium, and has mantle chemical signatures; it is called I-type granite. The other type, called S-type granite, has muscovite and biotite and is depleted in sodium but enriched in aluminum such that corundum occurs in the norm and isotopic signatures. This suggests that such granites were formed by partial fusion of sedimentary rocks.
Flood basalts
On the continental plates at areas away from active convergence, the magmatism is confined to rift valleys and local hot spots. The volume of magma produced is minor in comparison to that generated at oceanic rises and at convergent plate boundaries. Flood basalts are the most common form of occurrence. They span the rock record from the Precambrian to the Tertiary (from about 3.9 billion to 1.6 million years ago) and are found worldwide. The 1.1-billion-year-old Keweenawan flood basalts in the Lake Superior region of northern Michigan may have formed in a rift that failed. The rifting of Pangaea that began during Jurassic time (approximately 208 to 144 million years ago) generated flood basalt eruptions all along the newly opened Atlantic Ocean. Two voluminous eruptions associated with the opening of the South Atlantic produced the Paraná basalt in Brazil and the Karoo (or Karroo) in South Africa. The Deccan Plateau basalts in India were formed in the rift valleys associated with the breakup of Gondwana during the Cretaceous Period (approximately 144 to 66.4 million years ago). Chemically, the most abundant basalts are supersaturated tholeiites with normative quartz, but olivine tholeiites and alkali basalts also are found. Feeder dike swarms (groups consisting of many parallel dikes) and sills are common in flood basalt plateaus. Alkaline rocks, such as those found in the East African Rift System, occur as well but are less abundant. This rift system stretches southward from the Red Sea–Gulf of Aden to Lake Victoria. Undersaturated basalts are most common in these rifts. During one eruption, a magma composed mostly of sodium carbonate issued from a volcanic vent that had been erupting alkali basalts.
Other terrestrial occurrences
Other diverse and unusual igneous rocks are found in the stable continental areas far from plate boundaries. These include the large layered basaltic intrusions—namely, the Stillwater Complex in Montana, the Muskox intrusion in the Northwest Territories of Canada, the Bushveld Complex in South Africa, and the Skaergaard intrusion in eastern Greenland. Tholeiitic magma underwent a fractional crystallization process that deposited layers of ultramafic rocks overlain by gabbroic and anorthositic layers. The end products of this fractionation are quartz- and feldspar-bearing rocks with a peculiar texture (known as graphic intergrowth) in which quartz and feldspar are intimately intergrown with each other. These rocks are called granophyres (granophyre). Such layered intrusions have some economic importance; some of them contain thick (a few metres) layers of chromite, which is the source of chromium and also platinum. Two other rare occurrences in cratonic (stable) areas of the Earth's crust are the kimberlites (kimberlite) and carbonatites. Both are of economic value because they yield diamonds and niobium, respectively. Kimberlites are mica peridotites that are found in pipes. The stable interiors of South Africa and Siberia have widespread occurrences, but these pipes also are found in North America, Australia, Brazil, and India. In North America, near Murfreesboro, Ark., individuals can pay a fee to search for diamonds in the Prairie Creek kimberlite pipe located in the Crater of Diamonds State Park. Not all kimberlites contain diamonds. When diamonds do occur, they constitute less than one part per million of the rock. Carbonatites are igneous rocks rich in carbonate (containing at least 50 percent) that commonly occur in ring complexes in association with other silica-poor rocks such as nepheline syenites. In North America, carbonatites have been found in dozens of localities in northern Ontario and western Quebec.
Extraterrestrial occurrences
The dominant igneous rock on the Earth's surface is basalt. It appears that such is also the case on Earth's close neighbours. The lunar (Moon) maria (mare) are covered with basalt lava flows. These lunar basalts have a mineralogy similar to that of terrestrial basalts, but chemically they have no water, a lower amount of alkalis and alumina, and a higher iron oxide and chromium content. On the lunar highlands, plagioclase-rich rocks are most common; these include anorthosites, gabbros, troctolites (olivine-plagioclase rock), and minor basalt. It appears that basalt is common on Mars as well. The large shield volcano Olympus Mons must have been formed from eruptions of fluid basalt flows. The X-ray fluorescence analyses performed by the Vikings 1 and 2 landers showed that the rocks are basaltic. In contrast, compositions of meteorites that originated from Mars include both basalts and ultramafic rocks such as dunite, clinopyroxenite, and iherzolite. The Mars Pathfinder and Rover show that andesite may also be present, but that result is still debated. Venus apparently has volcanic features with granitic to basaltic compositions.
Additional Reading
Anthony Hall, Igneous Petrology, 2nd ed. (1996); Paul C. Hess, Origins of Igneous Rocks (1989); Anthony R. Philpotts, Principles of Igneous and Metamorphic Petrology (1990); Marjorie Wilson, Igneous Petrogenesis (1989); Robert Decker and Barbara Decker, Volcanoes, 3rd ed. (1998); Alexander R. McBirney, Igneous Petrology, 2nd ed. (1993); Jacques-Marie Bardintzeff and Alexander R. McBirney, Volcanology, 2nd ed. (2000).
- Mother Jones
- Mother Lode Country
- Mother Marie Joseph Butler
- Mother Mary Aloysia Hardey
- Mother's Day
- Mother Teresa Lalor
- Mother Théodore Guérin, Saint
- Mother Théodore, Saint Guérin
- Motherwell and Wishaw
- Motherwell, Robert
- moth fly
- moth orchid
- moth owl
- Motihari
- Motilal Nehru
- Motilón
- Mo, Timothy
- motion
- Motion, Andrew
- motion, equation of
- motion picture
- Motion Picture Arts and Sciences, Academy of
- Motion Picture Association of America
- motion-picture camera
- motion picture, history of the