lawrencium
chemical element
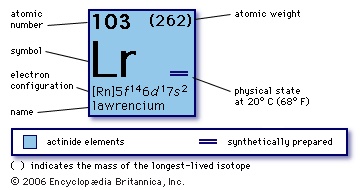 (Lr), synthetic chemical element, the 14th member of the actinoid series of the periodic table, atomic number 103. Not occurring in nature, lawrencium (as the isotopes lawrencium-257, lawrencium-258, and lawrencium-259) was produced (1961) by Albert Ghiorso, T. Sikkeland, A.E. Larsh, and R.M. Latimer at the University of California, Berkeley, by bombarding a mixture of the longest-lived isotopes of californium (atomic number 98) with boron ions (atomic number 5) accelerated in a heavy-ion linear accelerator. A team of Soviet scientists at the Joint Institute for Nuclear Research in Dubna discovered (1965) lawrencium-256 (35-second half-life), which the Berkeley group used to show that lawrencium behaves more like the tripositive elements in the actinide series than like predominantly dipositive nobelium (atomic number 102).
(Lr), synthetic chemical element, the 14th member of the actinoid series of the periodic table, atomic number 103. Not occurring in nature, lawrencium (as the isotopes lawrencium-257, lawrencium-258, and lawrencium-259) was produced (1961) by Albert Ghiorso, T. Sikkeland, A.E. Larsh, and R.M. Latimer at the University of California, Berkeley, by bombarding a mixture of the longest-lived isotopes of californium (atomic number 98) with boron ions (atomic number 5) accelerated in a heavy-ion linear accelerator. A team of Soviet scientists at the Joint Institute for Nuclear Research in Dubna discovered (1965) lawrencium-256 (35-second half-life), which the Berkeley group used to show that lawrencium behaves more like the tripositive elements in the actinide series than like predominantly dipositive nobelium (atomic number 102).atomic number
103
stablest isotope
256
oxidation state
+3
electronic config.
【Rn】5f146d17s2
- photography, technology of
- photo-ionization
- Photo League
- photoluminescence
- photolysis
- photometer
- photometry
- photomicrography
- photomontage
- photomultiplier tube
- photon
- photoperiodism
- photophore
- photoprotein
- Photo-realism
- photoreception
- photorecovery
- photorefractive keratectomy
- Photo-Secession
- photosensitization
- photosphere
- photosynthesis
- photovoltaic effect
- Phraates II
- Phraates III