americium
chemical element
(Am),
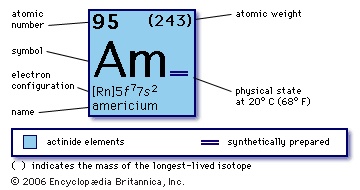 synthetic chemical element (atomic number 95) of the actinoid series of the periodic table. Undetected in nature, americium (as the isotope americium-241) was artificially produced from plutonium-239 (atomic number 94) in 1944 by Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso in a nuclear reactor. It was the fourth transuranium element to be discovered (curium, atomic number 96, was discovered a few months previously). The metal is silvery white and tarnishes slowly in dry air at room temperature. The isotope americium-241 is the most important because of its availability; it has been prepared in kilogram amounts from plutonium and has been used industrially in fluid-density gauges, thickness gauges, aircraft fuel gauges, and distance-sensing devices, all of which utilize its gamma radiation. The isotope's alpha-particle emission is exploited in smoke detectors. All isotopes of americium are radioactive; the stablest isotope, americium-243, has proved more convenient for chemical investigations in view of its longer half-life (7,370 years as compared with 458 years for americium-241).
synthetic chemical element (atomic number 95) of the actinoid series of the periodic table. Undetected in nature, americium (as the isotope americium-241) was artificially produced from plutonium-239 (atomic number 94) in 1944 by Glenn T. Seaborg, Ralph A. James, Leon O. Morgan, and Albert Ghiorso in a nuclear reactor. It was the fourth transuranium element to be discovered (curium, atomic number 96, was discovered a few months previously). The metal is silvery white and tarnishes slowly in dry air at room temperature. The isotope americium-241 is the most important because of its availability; it has been prepared in kilogram amounts from plutonium and has been used industrially in fluid-density gauges, thickness gauges, aircraft fuel gauges, and distance-sensing devices, all of which utilize its gamma radiation. The isotope's alpha-particle emission is exploited in smoke detectors. All isotopes of americium are radioactive; the stablest isotope, americium-243, has proved more convenient for chemical investigations in view of its longer half-life (7,370 years as compared with 458 years for americium-241).Americium reacts with oxygen to form the dioxide AmO2 and with hydrogen to form the hydride AmH2. There is some evidence that the ion Am2+ has been prepared in trace amounts, its existence suggesting that americium is similar to its lanthanoid homologue, europium, which can be reduced to its +2 oxidation state. Americium has four oxidation states, from +3 to +6, in acidic aqueous solution with the following ionic species: Am3+, pink; Am4+, rose (very unstable); AmO2 +, yellow; and AmO22+, light tan. In the common +3 state, americium is very similar to the other actinoid and lanthanoid elements.
atomic number
95
stablest isotope
243
melting point
above 850° C (1,550° F)
specific gravity
13.67 (20° C)
oxidation states
+2, +3, +4, +5, +6
electronic config.
【Rn】5f 77s2
- Glauber, Roy J.
- Glauber's salt
- glaucochroite
- glaucoma
- glauconite
- glaucophane
- glaucophane facies
- Glaucus
- Glavine, Tom
- glaze
- Glazov
- Glazunov, Aleksandr
- Gleason, Jackie
- Gleason, Kate
- Gleb Ivanovich Uspensky
- glee
- Gleicheniaceae
- Gleim, Johann Wilhelm Ludwig
- Glencairn, Alexander Cunningham, 5th earl of
- Whig and Tory
- Whig Party
- whimsey glass
- whinchat
- whipbird
- whiplash