lutetium
chemical element
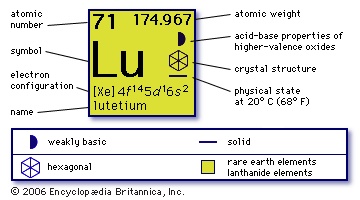 (Lu), chemical element, rare-earth metal of the lanthanoid series of the periodic table; the hardest and densest rare-earth element, last member of the lanthanoid series. Lutetium was discovered (1907–08) by Carl Auer von Welsbach (Welsbach, Carl Auer, Freiherr von) and Georges Urbain, working independently. Urbain derived the name for the element from Lutetia, which was the ancient Roman name for Paris. The name Urbain gave it to honour Paris, his native city, became widely accepted, except in Germany, where it was commonly called cassiopeium until the 1950s. One of the rarest of the rare earths, lutetium occurs in rare-earth minerals such as xenotime and euxenite. Though it composes only about 0.003 percent of the commercially important mineral monazite, it has proved feasible to extract it as a by-product. Separation and purification are accomplished by ion-exchange techniques. Lutetium is also found in the products of nuclear fission. The metal has been prepared by thermoreduction of the anhydrous halides by alkali or alkaline-earth metals. It has the highest melting point of the rare-earth elements. Natural lutetium consists of two isotopes: stable lutetium-175 (97.41 percent) and radioactive lutetium-176 (2.59 percent, 3 × 1010-year half-life). The radioactive isotope is used to determine the age of meteorites relative to that of the Earth. Few other uses have been found for lutetium.
(Lu), chemical element, rare-earth metal of the lanthanoid series of the periodic table; the hardest and densest rare-earth element, last member of the lanthanoid series. Lutetium was discovered (1907–08) by Carl Auer von Welsbach (Welsbach, Carl Auer, Freiherr von) and Georges Urbain, working independently. Urbain derived the name for the element from Lutetia, which was the ancient Roman name for Paris. The name Urbain gave it to honour Paris, his native city, became widely accepted, except in Germany, where it was commonly called cassiopeium until the 1950s. One of the rarest of the rare earths, lutetium occurs in rare-earth minerals such as xenotime and euxenite. Though it composes only about 0.003 percent of the commercially important mineral monazite, it has proved feasible to extract it as a by-product. Separation and purification are accomplished by ion-exchange techniques. Lutetium is also found in the products of nuclear fission. The metal has been prepared by thermoreduction of the anhydrous halides by alkali or alkaline-earth metals. It has the highest melting point of the rare-earth elements. Natural lutetium consists of two isotopes: stable lutetium-175 (97.41 percent) and radioactive lutetium-176 (2.59 percent, 3 × 1010-year half-life). The radioactive isotope is used to determine the age of meteorites relative to that of the Earth. Few other uses have been found for lutetium.The element behaves as a typical rare earth, forming a series of white salts in oxidation state +3, such as lutetium oxide, sulfate, and chloride.
atomic number
71
atomic weight
174.970
melting point
1,656° C
boiling point
3,315° C
specific gravity
9.835 (25° C)
oxidation state
+3
electronic config.
【Xe】4f145d16s2
- William Lawes
- William Le Baron Jenney
- William Lee
- William Lenthall
- William Lescaze
- William Lily
- William Lindley
- William Lisle Bowles
- William Lithgow
- William Livingston
- William Lloyd Garrison
- William Lloyd Garrison: The Dangers of Slavery (1829)
- William L Marcy
- William Longchamp
- William Longsword, 3rd earl of Salisbury
- William Longsword Salisbury, 3rd earl of
- William Lonsdale
- William Louis
- William Lovett
- William Lowndes Yancey
- William L. Shirer
- William Lyon Mackenzie
- William Lyon Phelps
- William Magear Tweed
- William Mahone