mercury
chemical element
Introduction
also called quicksilver
 chemical element, liquid metal of Group 12 (IIb, or zinc group (zinc group element)) of the periodic table.
chemical element, liquid metal of Group 12 (IIb, or zinc group (zinc group element)) of the periodic table.Properties, uses, and occurrence
Mercury was known in Egypt and also probably in the East as early as 1500 BC. The name mercury originated in 6th-century alchemy, in which the symbol of the planet was used to represent the metal; the chemical symbol Hg derives from the Latin hydrargyrum, “liquid silver.” Although its toxicity was recognized at an early date, its main application was for medical purposes.
Mercury is the only elemental metal that is liquid at room temperature. (Cesium melts at about 28.5 °C 【83 °F】, gallium at about 30 °C 【86 °F】, and rubidium at about 39 °C 【102 °F】.) Mercury is silvery white, slowly tarnishes in moist air, and freezes into a soft solid like tin or lead at about -39 °C (-38 °F). It boils at 357 °C (674 °F).
It alloys with copper, tin, and zinc to form amalgams, or liquid alloys. An amalgam with silver is used as a filling in dentistry. Mercury does not wet glass or cling to it, and this property, coupled with its rapid and uniform volume expansion throughout its liquid range, makes it useful in thermometers (thermometer). Barometers (barometer) and manometers utilize its high density and low vapour pressure. Gold and silver dissolve readily in mercury, and in the past this property was used in the extraction of these metals from their ores.
The good electrical conductivity of mercury makes it exceptionally useful in sealed electrical switches and relays. An electrical discharge through mercury vapour contained in a fused silica tube or bulb produces a bluish glow rich in ultraviolet light, a phenomenon exploited in ultraviolet, fluorescent, and high-pressure mercury-vapour lamps. Mercury's high thermal neutron-capture cross section (360 barns) and good thermal conductivity make it applicable as a shield and coolant in nuclear reactors. Much mercury is utilized in the preparation of pharmaceuticals and agricultural and industrial fungicides.
The use of mercury in the manufacture of chlorine and caustic soda (sodium hydroxide) by electrolysis of brine depends upon the fact that mercury employed as the negative pole, or cathode, dissolves the sodium liberated to form a liquid amalgam. An interesting application, though not of great commercial significance, is the use of mercury vapour instead of steam in some electrical generating plants, the higher boiling point of mercury providing greater efficiency in the heat cycle.
Mercury occurs in the Earth's crust on the average of about 0.08 gram (0.003 ounce) per ton of rock. The principal ore is the red sulfide, cinnabar. Native mercury occurs in isolated drops and occasionally in larger fluid masses, usually with cinnabar, near volcanoes or hot springs. Over half the world supply of mercury comes from Spain and Italy, and the free metal has been found in Serbia, at Mount Avala; in Slovenia, at Idrija; in Germany (Moschellandsberg); and in the United States (Terlingua, Texas, and New Almaden, Calif.). Cinnabar is mined in shaft or open-pit operations and refined by flotation. Most of the methods of extraction of mercury rely on the volatility of the metal and the fact that cinnabar is readily decomposed by air or by lime to yield the free metal. Because of the toxicity of mercury and the threat of rigid pollution control, attention is being directed toward safer methods of extracting mercury. These generally rely on the fact that cinnabar is readily soluble in solutions of sodium hypochlorite or sulfide, from which the mercury can be recovered by precipitation with zinc or aluminum or by electrolysis. (For treatment of the commercial production of mercury, see mercury processing; for mineralogical properties, see native element 【table】.)
Extremely rare natural alloys of mercury have also been found: moschellandsbergite (with silver), potarite (with palladium), and gold amalgam. Mercury is extracted from cinnabar by roasting it in air, followed by condensation of the mercury vapour. Mercury is toxic. Poisoning may result from inhalation of the vapour, ingestion of soluble compounds, or absorption of mercury through the skin.
Natural mercury is a mixture of seven stable isotopes: 196Hg (0.15 percent), 198Hg (10.02 percent), 199Hg (16.84 percent), 200Hg (23.13 percent), 201Hg (13.22 percent), 202Hg (29.80 percent), and 204Hg (6.85 percent). As a wavelength standard and for other precise work, isotopically pure mercury consisting of only mercury-198 is prepared by neutron bombardment of natural gold, gold-197.
Principal compounds
The compounds of mercury are either of +1 or +2 oxidation state. Mercury(II) or mercuric compounds predominate. Mercury does not combine with oxygen to produce mercury(II) oxide, HgO, at a useful rate until heated to the range of 300 to 350 °C (572 to 662 °F). At temperatures of about 400 °C (752 °F) and above, the reaction reverses with the compound decomposing into its elements. Antoine-Laurent Lavoisier and Joseph Priestley used this reaction in their study of oxygen.
There are relatively few mercury(I) or mercurous compounds. The mercury(I) ion, Hg22+, is diatomic and stable. Mercury(I) chloride, Hg2Cl2 (commonly known as calomel), is probably the most important univalent compound. It is used in antiseptic salves. Mercury(II) chloride, HgCl2 (also called bichloride of mercury or corrosive sublimate), is perhaps the commonest bivalent compound. Although extremely toxic, this odourless, colourless substance has a wide variety of applications. In agriculture it is used as a fungicide; in medicine it is sometimes employed as a topical antiseptic in concentrations of one part per 2,000 parts of water; and in the chemical industry it serves as a catalyst in the manufacture of vinyl chloride and as a starting material in the production of other mercury compounds. Mercury(II) oxide, HgO, provides elemental mercury for the preparation of various organic mercury compounds and certain inorganic mercury salts. This red or yellow crystalline solid is also used as an electrode (mixed with graphite) in zinc-mercuric oxide electric cells and in mercury batteries. Mercury(II) sulfide, HgS, is a black or red crystalline solid used chiefly as a pigment in paints, rubber, and plastics.
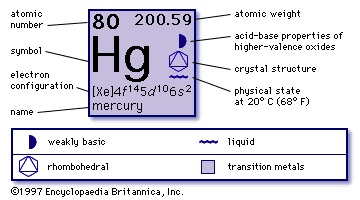
atomic number
80
atomic weight
200.59
melting point
-38.87 °C (-37.97 °F)
boiling point
356.9 °C (674 °F)
specific gravity
13.5 (20 °C 【68 °F】)
valence
1, 2
electronic config.
2-8-18-32-18-2 or (Xe)4f145d106s2
plant
 (genus Mercurialis), group of eight annual and perennial weedy flowering-plant species of the spurge family (Euphorbiaceae), native to Europe, Asia, and North Africa but naturalized in North America. Herb mercury (M. annua) grows as a weed in cultivated areas and shaded woods. Dog's mercury (M. perennis; see photograph-->
(genus Mercurialis), group of eight annual and perennial weedy flowering-plant species of the spurge family (Euphorbiaceae), native to Europe, Asia, and North Africa but naturalized in North America. Herb mercury (M. annua) grows as a weed in cultivated areas and shaded woods. Dog's mercury (M. perennis; see photograph--> ), which is malodorous and poisonous to livestock, grows wild in European woodlands. Its leaves are the source of an unstable blue dye. The clusters of small, green, nonpetaled male and female flowers are borne on separate plants; pollination is by wind. Three-seeded mercury, also in this family, is the annual weed Acalypha virginica.
), which is malodorous and poisonous to livestock, grows wild in European woodlands. Its leaves are the source of an unstable blue dye. The clusters of small, green, nonpetaled male and female flowers are borne on separate plants; pollination is by wind. Three-seeded mercury, also in this family, is the annual weed Acalypha virginica. - Manuel Gálvez
- Manuel I
- Manuel I Comnenus
- Manuel II
- Manuel II Palaeologus
- Manueline
- Manuelito
- Manuel José Quintana
- Manuel Lisa
- Manuel Lopes
- Manuel (Luis) Quezon (y Molina)
- Manuel Machado
- Manuel Maria Barbosa du Bocage
- Manuel Montt
- Manuel Moschopoulos
- Manuel Mujica Láinez
- Manuel, Niklaus
- Manuel Noriega
- Manuel Pavía y Lacy
- Manuel Pavía y Rodríguez de Alburquerque
- Manuel Puig
- Manuel Rojas
- Manuel Roxas
- Manuel Scorza
- Manuel Tamayo y Baus