metabolism
biology
Introduction
the sum of the chemical reactions (chemical reaction) that take place within each cell of a living organism and that provide energy for vital processes and for synthesizing new organic material.
Living organisms are unique in that they can extract energy from their environments and use it to carry out activities such as movement, growth and development, and reproduction. But how do living organisms—or, their cells—extract energy from their environments, and how do cells use this energy to synthesize and assemble the components from which the cells are made?
The answers to these questions lie in the enzyme-mediated chemical reactions that take place in living matter (metabolism). Hundreds of coordinated, multistep reactions, fueled by energy obtained from nutrients and/or solar energy, ultimately convert readily available materials into the molecules required for growth and maintenance.
The physical and chemical properties of the components of living things dealt with in this article are found in the articles carbohydrate; cell; hormone; lipid; photosynthesis; and protein.
A summary of metabolism
The unity of life
At the cellular level of organization, the main chemical (biochemistry) processes of all living matter are similar, if not identical. This is true for animals, plants, fungi, or bacteria; where variations occur (such as, for example, in the secretion of antibodies by some molds), the variant processes are but variations on common themes. Thus, all living matter is made up of large molecules called proteins (protein), which provide support and coordinated movement, as well as storage and transport of small molecules, and, as catalysts, enable chemical reactions to take place rapidly and specifically under mild temperature, relatively low concentration, and neutral conditions (i.e., neither acidic nor basic). Proteins are assembled from some 20 amino acids (amino acid), and, just as the 26 letters of the alphabet can be assembled in specific ways to form words of various lengths and meanings, so may tens or even hundreds of the 20 amino-acid “letters” be joined to form specific proteins. Moreover, those portions of protein molecules involved in performing similar functions in different organisms often comprise the same sequences of amino acids.
There is the same unity among cells of all types in the manner in which living organisms preserve their individuality and transmit it to their offspring. For example, hereditary information is encoded in a specific sequence of bases that make up the DNA (deoxyribonucleic acid) molecule in the nucleus of each cell. Only four bases are used in synthesizing DNA: adenine, guanine, cytosine, and thymine. Just as the Morse Code consists of three simple signals—a dash, a dot, and a space—the precise arrangement of which suffices to convey coded messages, so the precise arrangement of the bases in DNA contains and conveys the information for the synthesis and assembly of cell components. Some primitive life-forms, however, use RNA (ribonucleic acid; a nucleic acid differing from DNA in containing the sugar ribose instead of the sugar deoxyribose and the base uracil instead of the base thymine) in place of DNA as a primary carrier of genetic information. The replication of the genetic material in these organisms must, however, pass through a DNA phase. With minor exceptions, the genetic code used by all living organisms is the same.
The chemical reactions that take place in living cells are similar as well. Green plants use the energy of sunlight (photosynthesis) to convert water (H2O) and carbon dioxide (CO2) to carbohydrates (carbohydrate) (sugars and starches), other organic (carbon-containing) compounds, and molecular oxygen (O2). The process of photosynthesis requires energy, in the form of sunlight, to split one water molecule into one-half of an oxygen molecule (O2; the oxidizing agent) and two hydrogen atoms (H; the reducing agent), each of which dissociates to one hydrogen ion (H+) and one electron. Through a series of oxidation-reduction reactions, electrons (denoted e-) are transferred from a donating molecule (oxidation), in this case water, to an accepting molecule (reduction) by a series of chemical reactions; this “reducing power” may be coupled ultimately to the reduction of carbon dioxide to the level of carbohydrate. In effect, carbon dioxide accepts and bonds with hydrogen, forming carbohydrates (Cn【H2O】n).
Living organisms that require oxygen reverse this process: they consume carbohydrates and other organic materials, using oxygen synthesized by plants to form water, carbon dioxide, and energy. The process that removes hydrogen atoms (containing electrons) from the carbohydrates and passes them to the oxygen is an energy-yielding series of reactions.
In plants, all but two of the steps in the process that converts carbon dioxide to carbohydrates are the same as those steps that synthesize sugars from simpler starting materials in animals, fungi, and bacteria. Similarly, the series of reactions that take a given starting material and synthesize certain molecules that will be used in other synthetic pathways are similar, or identical, among all cell types. From a metabolic point of view, the cellular processes that take place in a lion are only marginally different from those that take place in a dandelion.
Biological energy exchanges
The energy changes associated with physicochemical processes are the province of thermodynamics, a subdiscipline of physics. The first two laws of thermodynamics state, in essence, that energy can be neither created nor destroyed and that the effect of physical and chemical changes is to increase the disorder, or randomness (i.e., entropy), of the universe. Although it might be supposed that biological processes—through which organisms grow in a highly ordered and complex manner, maintain order and complexity throughout their life, and pass on the instructions for order to succeeding generations—are in contravention of these laws, this is not so. Living organisms neither consume nor create energy: they can only transform it from one form to another. From the environment they absorb energy in a form useful to them; to the environment they return an equivalent amount of energy in a biologically less useful form. The useful energy, or free energy, may be defined as energy capable of doing work under isothermal conditions (conditions in which no temperature differential exists); free energy is associated with any chemical change. Energy less useful than free energy is returned to the environment, usually as heat. Heat cannot perform work in biological systems because all parts of cells have essentially the same temperature and pressure.
The carrier of chemical energy
At any given time, a neutral molecule of water dissociates into a hydrogen ion (H+) and a hydroxide ion (OH-), and the ions are continually re-forming into the neutral molecule. Under normal conditions (neutrality), the concentration of hydrogen ions (acidic ions) is equal to that of the hydroxide ions (basic ions); each are at a concentration of 10-7 moles per litre, which is described as a pH of 7.
 All cells either are bounded by membranes or contain organelles that have membranes. These membranes do not permit water or the ions derived from water to pass into or out of the cells or organelles. In green plants, sunlight is absorbed by chlorophyll and other pigments in the chloroplasts of the cells, called photosystem II. As shown previously, when a water molecule is split by light energy, one-half of an oxygen molecule and two hydrogen atoms (which dissociate to two electrons and two hydrogen ions, H+) are formed. When excited by sunlight, chlorophyll loses one electron to an electron carrier molecule but quickly recovers it from a hydrogen atom of the split water molecule, which sends H+ into solution in the process. Two oxygen atoms come together to form a molecule of oxygen gas (O2). The free electrons are passed to photosystem I, but, in doing so, an excess concentration of positively charged hydrogen ions (H+) appears on one side of the membrane in the chloroplast, whereas an excess of negatively charged hydroxide ions (OH-) builds up on the other side. The free energy released as H+ ions move through a specific “pore” in the membrane, to equalize the concentrations of ions, is sufficient to make some biological processes work, such as the uptake of certain nutrients by bacteria and the rotation of the whiplike protein-based propellers that enable such bacteria to move. Equally important, however, is that this gradient across the membrane powers the formation of adenosine triphosphate (ATP) from inorganic phosphate (HPO42-, abbreviated Pi) and adenosine diphosphate (ADP). It is ATP (Figure 1-->
All cells either are bounded by membranes or contain organelles that have membranes. These membranes do not permit water or the ions derived from water to pass into or out of the cells or organelles. In green plants, sunlight is absorbed by chlorophyll and other pigments in the chloroplasts of the cells, called photosystem II. As shown previously, when a water molecule is split by light energy, one-half of an oxygen molecule and two hydrogen atoms (which dissociate to two electrons and two hydrogen ions, H+) are formed. When excited by sunlight, chlorophyll loses one electron to an electron carrier molecule but quickly recovers it from a hydrogen atom of the split water molecule, which sends H+ into solution in the process. Two oxygen atoms come together to form a molecule of oxygen gas (O2). The free electrons are passed to photosystem I, but, in doing so, an excess concentration of positively charged hydrogen ions (H+) appears on one side of the membrane in the chloroplast, whereas an excess of negatively charged hydroxide ions (OH-) builds up on the other side. The free energy released as H+ ions move through a specific “pore” in the membrane, to equalize the concentrations of ions, is sufficient to make some biological processes work, such as the uptake of certain nutrients by bacteria and the rotation of the whiplike protein-based propellers that enable such bacteria to move. Equally important, however, is that this gradient across the membrane powers the formation of adenosine triphosphate (ATP) from inorganic phosphate (HPO42-, abbreviated Pi) and adenosine diphosphate (ADP). It is ATP (Figure 1--> ) that is the major carrier of biologically utilizable energy in all forms of living matter. The interrelationships of energy-yielding and energy-requiring metabolic reactions may be considered largely as processes that couple the formation of ATP with its breakdown.
) that is the major carrier of biologically utilizable energy in all forms of living matter. The interrelationships of energy-yielding and energy-requiring metabolic reactions may be considered largely as processes that couple the formation of ATP with its breakdown.Synthesis of ATP by green plants is similar to the synthesis of ATP that takes place in the mitochondria of animal, plant, and fungus cells, and in the plasma membranes of bacteria that use oxygen (or other inorganic electron acceptors, such as nitrate) to accept electrons from the removal of hydrogen atoms from a molecule of food (see below The combustion of food materials: Biological energy transduction (metabolism)). Through these processes most of the energy stored in food materials is released and converted into the molecules that fuel life processes. It must also be remembered, however, that many living organisms (usually bacteria and protozoa) cannot tolerate oxygen; they form ATP from inorganic phosphate and ADP by substrate-level phosphorylations (the addition of a phosphate group) that do not involve the establishment and collapse of proton gradients across membranes. Such processes are discussed in detail below (The fragmentation of complex molecules: The catabolism of glucose (metabolism)). It must also be borne in mind that the fuels of life and the cellular “furnace” in which they are “burned” are made of the same types of material: if the fires burn too brightly, not only the fuel but also the furnace is consumed. It is therefore essential to release energy at small, discrete, readily utilizable intervals. The relative complexity of the catabolic pathways (by which food materials are broken down) and the complexity of the anabolic pathways (by which cell components are synthesized) reflect this need and offer the possibility for simple feedback systems to control the rate at which materials travel along these sequences of enzymic reactions.
catabolism
Formation of small molecules. The release of chemical energy from food materials essentially occurs in three phases. In the first phase (phase I), the large molecules that make up the bulk of food materials are broken down into small constituent units: proteins are converted to the 20 or so different amino acids of which they are composed; carbohydrates (polysaccharides such as starch in plants and glycogen in animals) are degraded to sugars such as glucose; and fats (lipids) are broken down into fatty acids and glycerol. The amounts of energy liberated in phase I are relatively small: only about 0.6 percent of the free, or useful, energy of proteins and carbohydrates, and about 0.1 percent of that of fats, is released during this phase. Because this energy is liberated largely as heat, it cannot be utilized by the cell. The purpose of the reactions of phase I, which can be grouped under the term digestion and which, in animals, occur mainly in the intestinal tract and in tissues in which reserve materials are prepared, or mobilized, for energy production, is to prepare the foodstuffs for the energy-releasing processes.
Incomplete oxidation

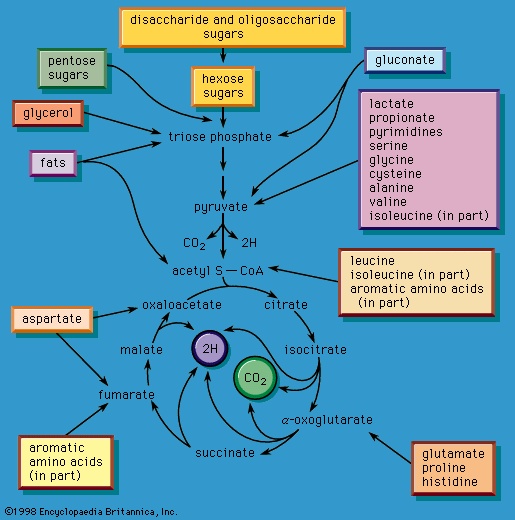 In the second phase of the release of energy from food (phase II), the small molecules produced in the first phase—sugars, glycerol, a number of fatty acids, and about 20 varieties of amino acids—are incompletely oxidized (in this sense, oxidation means the removal of electrons or hydrogen atoms), the end product being (apart from carbon dioxide and water) one of only three possible substances: the two-carbon compound acetate, in the form of a compound called acetyl coenzyme A (Figure 1-->
In the second phase of the release of energy from food (phase II), the small molecules produced in the first phase—sugars, glycerol, a number of fatty acids, and about 20 varieties of amino acids—are incompletely oxidized (in this sense, oxidation means the removal of electrons or hydrogen atoms), the end product being (apart from carbon dioxide and water) one of only three possible substances: the two-carbon compound acetate, in the form of a compound called acetyl coenzyme A (Figure 1--> ); the four-carbon compound oxaloacetate; and the five-carbon compound α-oxoglutarate. The first, acetate in the form of acetyl coenzyme A, constitutes by far the most common product—it is the product of two-thirds of the carbon incorporated into carbohydrates and glycerol; all of the carbon in most fatty acids; and approximately half of the carbon in amino acids. The end product of several amino acids is α-oxoglutarate; that of a few others is oxaloacetate, which is formed either directly or indirectly (from fumarate). These processes, represented diagrammatically in Figure 2-->
); the four-carbon compound oxaloacetate; and the five-carbon compound α-oxoglutarate. The first, acetate in the form of acetyl coenzyme A, constitutes by far the most common product—it is the product of two-thirds of the carbon incorporated into carbohydrates and glycerol; all of the carbon in most fatty acids; and approximately half of the carbon in amino acids. The end product of several amino acids is α-oxoglutarate; that of a few others is oxaloacetate, which is formed either directly or indirectly (from fumarate). These processes, represented diagrammatically in Figure 2--> , show what happens in the bacterium Escherichia coli, but essentially similar processes occur in animals, plants, fungi, and other organisms capable of oxidizing their food materials wholly to carbon dioxide and water.
, show what happens in the bacterium Escherichia coli, but essentially similar processes occur in animals, plants, fungi, and other organisms capable of oxidizing their food materials wholly to carbon dioxide and water.Complete oxidation
Total oxidation of the relatively few products of phase II occurs in a cyclic sequence of chemical reactions known as the tricarboxylic acid (TCA) cycle, or the Krebs cycle (tricarboxylic acid cycle), after its discoverer, Sir Hans Krebs; it represents phase III of energy release from foods. Each turn of this cycle (see below The tricarboxylic acid 【TCA】 cycle (metabolism)) is initiated by the formation of citrate, with six carbon atoms, from oxaloacetate (with four carbons) and acetyl coenzyme A; subsequent reactions result in the reformation of oxaloacetate and the formation of two molecules of carbon dioxide. The carbon atoms that go into the formation of carbon dioxide are no longer available to the cell. The concomitant stepwise oxidations—in which hydrogen atoms or electrons are removed from intermediate compounds formed during the cycle and, via a system of carriers, are transferred ultimately to oxygen to form water—are quantitatively the most important means of generating ATP from ADP and inorganic phosphate. These events are known as terminal respiration and oxidative phosphorylation (for details of this process, see below Biological energy transduction (metabolism)).
Some microorganisms, incapable of completely converting their carbon compounds to carbon dioxide, release energy by fermentation reactions, in which the intermediate compounds of catabolic routes either directly or indirectly accept or donate hydrogen atoms. Such secondary changes in intermediate compounds result in considerably less energy being made available to the cell than occurs with the pathways that are linked to oxidative phosphorylation; however, fermentation reactions yield a large variety of commercially important products. Thus, for example, if the oxidation (removal of electrons or hydrogen atoms) of some catabolic intermediate is coupled to the reduction of pyruvate or of acetaldehyde derived from pyruvate, the products formed are lactic acid and ethyl alcohol, respectively.
anabolism
 Catabolic pathways effect the transformation of food materials into the interconvertible intermediates of the pathways shown in Figure 2-->
Catabolic pathways effect the transformation of food materials into the interconvertible intermediates of the pathways shown in Figure 2--> . Anabolic pathways, on the other hand, are sequences of enzyme-catalyzed reactions in which the component building blocks of large molecules, or macromolecules (e.g., proteins, carbohydrates, and fats), are constructed from the same intermediates. Thus, catabolic routes have clearly defined beginnings but no unambiguously identifiable end products; anabolic routes, on the other hand, lead to clearly distinguishable end products from diffuse beginnings. The two types of pathway are linked through reactions of phosphate transfer, involving ADP, AMP, and ATP as described above, and also through electron transfers, which enable reducing equivalents (i.e., hydrogen atoms or electrons), which have been released during catabolic reactions, to be utilized for biosynthesis. But, although catabolic and anabolic pathways are closely linked, and although the overall effect of one type of route is obviously the opposite of the other, they have few steps in common. The anabolic pathway for the synthesis of a particular molecule generally starts from intermediate compounds quite different from those produced as a result of catabolism of that molecule; for example, microorganisms catabolize aromatic (i.e., containing a ring, or cyclic, structure) amino acids to acetyl coenzyme A and an intermediate compound of the TCA cycle. The biosynthesis of these amino acids, however, starts with a compound derived from pyruvate and an intermediate compound of the metabolism of pentose (a general name for sugars with five carbon atoms). Similarly, histidine is synthesized from a pentose sugar but is catabolized to α-oxoglutarate.
. Anabolic pathways, on the other hand, are sequences of enzyme-catalyzed reactions in which the component building blocks of large molecules, or macromolecules (e.g., proteins, carbohydrates, and fats), are constructed from the same intermediates. Thus, catabolic routes have clearly defined beginnings but no unambiguously identifiable end products; anabolic routes, on the other hand, lead to clearly distinguishable end products from diffuse beginnings. The two types of pathway are linked through reactions of phosphate transfer, involving ADP, AMP, and ATP as described above, and also through electron transfers, which enable reducing equivalents (i.e., hydrogen atoms or electrons), which have been released during catabolic reactions, to be utilized for biosynthesis. But, although catabolic and anabolic pathways are closely linked, and although the overall effect of one type of route is obviously the opposite of the other, they have few steps in common. The anabolic pathway for the synthesis of a particular molecule generally starts from intermediate compounds quite different from those produced as a result of catabolism of that molecule; for example, microorganisms catabolize aromatic (i.e., containing a ring, or cyclic, structure) amino acids to acetyl coenzyme A and an intermediate compound of the TCA cycle. The biosynthesis of these amino acids, however, starts with a compound derived from pyruvate and an intermediate compound of the metabolism of pentose (a general name for sugars with five carbon atoms). Similarly, histidine is synthesized from a pentose sugar but is catabolized to α-oxoglutarate.Even in cases in which a product of catabolism is used in an anabolic pathway, differences emerge; thus, fatty acids, which are catabolized to acetyl coenzyme A, are synthesized not from acetyl coenzyme A directly but from a derivative of it, malonyl coenzyme A (see below The biosynthesis of cell components: Lipid components (metabolism)). Furthermore, even enzymes that catalyze apparently identical steps in catabolic and anabolic routes may exhibit different properties. In general, therefore, the way down (catabolism) is different from the way up (anabolism). These differences are important because they allow for the regulation of catabolic and anabolic processes in the cell.
In eukaryotic (eukaryote) cells (i.e., those with a well-defined nucleus, characteristic of organisms higher than bacteria) the enzymes of catabolic and anabolic pathways are often located in different cellular compartments. This also contributes to the manner of their cellular control; for example, the formation of acetyl coenzyme A from fatty acids, referred to above, occurs in animal cells in small sausage-shaped components, or organelles, called mitochondria (mitochondrion), which also contain the enzymes for terminal respiration and for oxidative phosphorylation. The biosynthesis of fatty acids from acetyl coenzyme A, on the other hand, occurs in the cytoplasm.
Integration of catabolism and anabolism
Fine control
Possibly the most important means for controlling the flux of metabolites through catabolic and anabolic pathways, and for integrating the numerous different pathways in the cell, is through the regulation of either the activity or the synthesis of key (pacemaker) enzymes (enzyme). It was recognized in the 1950s, largely from work with microorganisms, that pacemaker enzymes can interact with small molecules at more than one site on the surface of the enzyme molecule. The reaction between an enzyme and its substrate—defined as the compound with which the enzyme acts to form a product—occurs at a specific site on the enzyme known as the catalytic, or active, site; the proper fit between the substrate and the active site is an essential prerequisite for the occurrence of a reaction catalyzed by an enzyme. Interactions at other, so-called regulatory sites on the enzyme, however, do not result in a chemical reaction but cause changes in the shape of the protein; the changes profoundly affect the catalytic properties of the enzyme, either inhibiting or stimulating the rate of the reaction. Modulation of the activity of pacemaker enzymes may be effected by metabolites of the pathway in which the enzyme acts or by those of another pathway; the process may be described as a “fine control” of metabolism. Very small changes in the chemical environment thus produce important and immediate effects on the rates at which individual metabolic processes occur.
Most catabolic pathways are regulated by the relative proportions of ATP, ADP, and AMP in the cell. It is reasonable to suppose that a pathway that serves to make ATP available for energy-requiring reactions would be less active if sufficient ATP were already present, than if ADP or AMP were to accumulate. The relative amounts of the adenine nucleotides (i.e., ATP, ADP, and AMP) thus modulate the overall rate of catabolic pathways. They do so by reacting with specific regulatory sites on pacemaker enzymes necessary for the catabolic pathways, which do not participate in the anabolic routes that effect the opposite reactions. Similarly, it is reasonable to suppose that many anabolic processes, which require energy, are inhibited by ADP or AMP; elevated levels of these nucleotides may be regarded therefore as cellular distress signals indicating a lack of energy.
Since one way in which anabolic pathways differ from catabolic routes is that the former result in identifiable end products, it is not unexpected that the pacemaker enzymes of many anabolic pathways—particularly those effecting the biosynthesis of amino acids and nucleotides —are regulated by the end products of these pathways or, in cases in which branching of pathways occurs, by end products of each branch. Such pacemaker enzymes usually act at the first step unique to a particular anabolic route. If branching occurs, the first step of each branch is controlled. By this so-called negative feedback system, the cellular concentrations of products determine the rates of their formation, thus ensuring that the cell synthesizes only as much of the products as it needs.
Coarse control
A second and less immediately responsive, or “coarse,” control is exerted over the synthesis of pacemaker enzymes. The rate of protein synthesis reflects the activity of appropriate genes, which contain the information that directs all cellular processes. Coarse control is therefore exerted on genetic material rather than on enzymes. Preferential synthesis of a pacemaker enzyme is particularly required to accommodate a cell to major changes in its chemical milieu. Such changes occur in multicellular organisms only to a minor extent, so that this type of control mechanism is less important in animals than in microorganisms. In the latter, however, it may determine the ease with which a cell previously growing in one nutrient medium can grow after transfer to another. In cases in which several types of organism compete in the same medium for available carbon sources, the operation of coarse controls may well be decisive in ensuring survival.
Alterations in the differential rates of synthesis of pacemaker enzymes in microorganisms responding to changes in the composition of their growth medium also manifest the properties of negative feedback systems. Depending on the nature of the metabolic pathway of which a pacemaker enzyme is a constituent, the manner in which the alterations are elicited may be distinguished. Thus, an increase in the rates at which enzymes of catabolic routes are synthesized results from the addition of inducers—usually compounds that exhibit some structural similarity to the substrates on which the enzymes act. A classic example of an inducible enzyme of this type is β-galactosidase. Escherichia coli growing in nutrient medium containing glucose do not utilize the milk sugar, lactose (glucose-4-β-d-galactoside); however, if the bacteria are placed in a growth medium containing lactose as the sole source of carbon, they synthesize β-galactosidase and can therefore utilize lactose. The reaction catalyzed by the enzyme is the hydrolysis (i.e., breakdown involving water) of lactose to its two constituent sugars, glucose and galactose; the preferential synthesis of the enzyme thus allows the bacteria to use the lactose for growth and energy. Another characteristic of the process of enzyme induction is that it continues only as long as the inducer (in this case, lactose) is present; if cells synthesizing β-galactosidase are transferred to a medium containing no lactose, synthesis of β-galactosidase ceases, and the amount of the enzyme in the cells is diluted as they divide, until the original low level of the enzyme is reestablished.
In contrast, the differential rates of synthesis of pacemaker enzymes of anabolic routes are usually not increased by the presence of inducers. Instead, the absence of small molecules that act to repress enzyme synthesis accelerates enzyme formation. Similar to the fine control processes described above is the regulation by coarse control of many pacemaker enzymes of amino-acid biosynthesis. Like the end product inhibitors, the repressors in these cases also appear to be the amino-acid end products themselves.
It is useful to regard the acceleration of the enzyme-forming machinery as the consequence, metaphorically, of either placing a foot on the accelerator or removing it from the brake. Analysis of the mechanisms by which gene activity is controlled suggest, however, that the distinction between inducible and repressible enzymes may be more apparent than real (see below Regulation of metabolism (metabolism)).
The study of metabolic pathways
There are two main reasons for studying a metabolic pathway: (1) to describe, in quantitative terms, the chemical changes catalyzed by the component enzymes of the route; and (2) to describe the various intracellular controls that govern the rate at which the pathway functions.
Studies with whole organisms or organs can provide information that one substance is converted to another and that this process is localized in a certain tissue; for example, experiments can show that urea, the chief nitrogen-containing end product of protein metabolism in mammals, is formed exclusively in the liver. They cannot reveal, however, the details of the enzymatic steps involved. Clues to the identity of the products involved, and to the possible chemical changes effected by component enzymes, can be provided in any of four ways involving studies with either whole organisms or tissues.
First, under stress or the imbalances associated with diseases, certain metabolites may accumulate to a greater extent than normal. Thus, during the stress of violent exercise, lactic acid appears in the blood, while glycogen, the form in which carbohydrate is stored in muscle, disappears. Such observations do not, however, prove that lactic acid is a normal intermediate of glycogen catabolism; rather, they show only that compounds capable of yielding lactic acid are likely to be normal intermediates. Indeed, in the example, lactic acid is formed in response to abnormal circumstances and is not directly formed in the pathways of carbohydrate catabolism. On the other hand, the abnormal accumulation of pyruvic acid in the blood of vitamin B1-deficient pigeons was a valuable clue to the role of this vitamin in the oxidation of pyruvate.
Second, the administration of metabolic poisons may lead to the accumulation of specific metabolites. If fluoroacetic acid or fluorocitric acid is ingested by animals, for example, citric acid accumulates in the liver. This correctly suggests that fluorocitric acid administered as such, or formed from fluoroacetic acid via the tricarboxylic acid (TCA) cycle, inhibits an enzyme of citrate oxidation.
Third, the fate of any nutrient—indeed, often the fate of a particular chemical group or atom in a nutrient—can be followed with relative ease by administering the nutrient labeled with an isotope. Isotopes are forms of an element that are chemically indistinguishable from each other but differ in physical properties.
The use of a nonradioactive isotope of nitrogen in the 1930s first revealed the dynamic state of body constituents. It had previously been believed that the proteins of tissues (tissue) are stable once formed, disappearing only with the death of the cell. By feeding amino acids labeled with isotopic nitrogen to rats, it was discovered that the isotope was incorporated into many of the amino acids found in proteins of the liver and the gut, even though the total protein content of these tissues did not change. This suggested that the proteins of these tissues exist in a dynamic steady state, in which relatively high rates of synthesis are counterbalanced by equal rates of degradation. Thus, although the average liver cell has a life-span of several months, half of its proteins are synthesized and degraded every five to six days. On the other hand, the proteins of the muscle or the brain, tissues that (unlike the gut or liver) need not adjust to changes in the chemical composition of their milieu, do not turn over as rapidly. The high rates of turnover observed in liver and gut tissues indicate that the coarse controls, exerted through the onset and cessation of synthesis of pacemaker enzymes, do occur in animal cells.
Finally, genetically altered organisms (mutants (mutation)) fail to synthesize certain enzymes in an active form. Such defects, if not lethal, result in the accumulation and excretion of the substrate of the defective enzyme; in normal organisms, the substrate would not accumulate, because it would be acted upon by the enzyme. The significance of this observation was first realized in the early 20th century when the phrase “inborn errors of metabolism” was used to describe hereditary conditions in which a variety of amino acids and other metabolites are excreted in the urine. In microorganisms, in which it is relatively easy to cause genetic mutations and to select specific mutants, this technique has been very useful. In addition to their utility in the unraveling of metabolic pathways, the use of mutants in the early 1940s led to the postulation of the one gene-one enzyme hypothesis by the Nobel Prize winners George W. Beadle (Beadle, George Wells) and Edward L. Tatum (Tatum, Edward L.); their discoveries opened the field of biochemical genetics and first revealed the nature of the fine controls of metabolism.
Because detailed information about the mechanisms of component enzymatic steps in any metabolic pathway cannot be obtained from studies with whole organisms or tissues, various techniques have been developed for studying these processes—e.g., sliced tissues, and homogenates and cell-free extracts, which are produced by physical disruption of the cells and the removal of cell walls and other debris. The sliced-tissue technique was successfully used by the Nobel Prize winner Sir Hans Krebs (Krebs, Sir Hans Adolf) in his pioneer studies in the early 1930s on the mechanism of urea formation in the liver. Measurements were made of the stimulating effects of small quantities of amino acids on both the rate of oxygen uptake and the amount of oxygen taken up; the amino acids were added to liver slices bathed in a nutrient medium. Such measurements revealed the cyclic nature of the process; specific amino acids acted as catalysts, stimulating respiration to an extent greater than expected from the quantities added. This was because the added material had been re-formed in the course of the cycle (see below The catabolism of proteins: Disposal of nitrogen (metabolism)).
Homogenates of tissue are useful in studying metabolic processes because permeability barriers that may prevent ready access of external materials to cell components are destroyed. The tissue is usually minced, blended, or otherwise disrupted in a medium that is suitably buffered to maintain the normal acid–base balance of the tissue, and contains the ions required for many life processes, chiefly sodium, potassium, and magnesium. The tissue is either used directly—as was done by Krebs in elucidating, in 1937, the TCA cycle from studies of the respiration of minced pigeon breast muscle—or fractionated (i.e., broken down) further. If the latter procedure is followed, homogenization is often carried out in a medium containing a high concentration of the sugar sucrose, which provides a milieu favourable for maintaining the integrity of cellular components. The components are recovered by careful spinning in a centrifuge, at a series of increasing speeds. It is thus possible to obtain fractions containing predominantly one type of organelle: nuclei (and some unbroken cells); mitochondria, lysosomes, and microbodies; microsomes (i.e., ribosomes and endoplasmic reticulum fragments); and—after prolonged centrifugation at forces in excess of 100,000 times gravity—a clear liquid that represents the soluble fraction of the cytoplasm. The fractions thus obtained can be further purified and tested for their capacity to carry out a given metabolic step or steps. This procedure was used to show that isolated mitochondria catalyze the oxidation reactions of the TCA cycle and that these organelles also contain the enzymes of fatty acid oxidation. Similarly, isolated ribosomes are used to study the pathway and mechanism of protein synthesis.
The final step in elucidating a reaction in a metabolic pathway includes isolation of the enzyme involved. The rate of the reaction and the factors that control the activity of the enzyme are then measured.
It should be emphasized that biochemists realize that studies on isolated and highly purified systems, such as those briefly described above, can do no more than approximate biological reality. The identification of the fine and coarse controls of a metabolic pathway, and (when appropriate) other influences on that pathway, must ultimately involve the study of the pathway in the whole cell or organism. Although some techniques have proved adequate for relating findings in the test tube to the situation in living organisms, study of the more complex metabolic processes, such as those involved in differentiation and development, may require the elaboration of new experimental approaches.
The fragmentation of complex molecules (catabolism)
Food materials must undergo oxidation in order to yield biologically useful energy. Oxidation does not necessarily involve oxygen, although it must involve the transfer of electrons from a donor molecule to a suitable acceptor molecule; the donor is thus oxidized and the recipient reduced. Many microorganisms either must live in the absence of oxygen (i.e., are obligate anaerobes) or can live in its presence or its absence (i.e., are facultative anaerobes).
If no oxygen is available, the catabolism of food materials is effected via fermentations, in which the final acceptor of the electrons removed from the nutrient is some organic molecule, usually generated during the fermentation process. There is no net oxidation of the food molecule in this type of catabolism; that is, the overall oxidation state of the fermentation products is the same as that of the starting material.
Organisms that can use oxygen as a final electron acceptor also use many of the steps in the fermentation pathways in which food molecules are broken down to smaller fragments; these fragments, instead of serving as electron acceptors, are fed into the TCA cycle, the pathway of terminal respiration.
In this cycle all of the hydrogen atoms (H) or electrons (e-) are removed from the fragments and are channeled through a series of electron carriers, ultimately to react with oxygen (O; see below Energy conservation (metabolism)). All carbon atoms are eliminated as carbon dioxide (CO2) in this process. The sequence of reactions involved in the catabolism of food materials may thus be conveniently considered in terms of an initial fragmentation (fermentation), followed by a combustion (respiration) process.
The catabolism of glucose
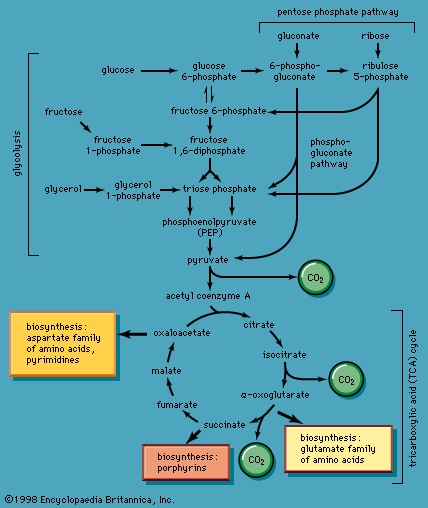
Glycolysis
The transformation of glucose. Quantitatively, the most important source of energy for cellular processes is the six-carbon sugar glucose (C6H12O6). Two structures of glucose are shown in Figure 3--> , in which the carbon atoms are numbered. (See carbohydrate for a discussion of the chemical nature of glucose and other carbohydrates.) Glucose is made available to animals through the hydrolysis of polysaccharides, such as glycogen and starch, the process being catalyzed by digestive enzymes. In animals, the sugar thus set free passes from the gut into the bloodstream and from there into the cells of the liver and other tissues. In microorganisms, of course, no such specialized tissues are involved.
, in which the carbon atoms are numbered. (See carbohydrate for a discussion of the chemical nature of glucose and other carbohydrates.) Glucose is made available to animals through the hydrolysis of polysaccharides, such as glycogen and starch, the process being catalyzed by digestive enzymes. In animals, the sugar thus set free passes from the gut into the bloodstream and from there into the cells of the liver and other tissues. In microorganisms, of course, no such specialized tissues are involved.
 , in which the carbon atoms are numbered. (See carbohydrate for a discussion of the chemical nature of glucose and other carbohydrates.) Glucose is made available to animals through the hydrolysis of polysaccharides, such as glycogen and starch, the process being catalyzed by digestive enzymes. In animals, the sugar thus set free passes from the gut into the bloodstream and from there into the cells of the liver and other tissues. In microorganisms, of course, no such specialized tissues are involved.
, in which the carbon atoms are numbered. (See carbohydrate for a discussion of the chemical nature of glucose and other carbohydrates.) Glucose is made available to animals through the hydrolysis of polysaccharides, such as glycogen and starch, the process being catalyzed by digestive enzymes. In animals, the sugar thus set free passes from the gut into the bloodstream and from there into the cells of the liver and other tissues. In microorganisms, of course, no such specialized tissues are involved.The fermentative phase of glucose catabolism (glycolysis) involves several enzymes; the action of each is summarized below. In living cells many of the compounds that take part in metabolism exist as negatively charged moieties, or anions, and are named as such in most of this article; e.g., pyruvate, oxaloacetate.
In order to obtain a net yield of ATP from the catabolism of glucose, it is first necessary to invest ATP. During step 【1--> 】 the alcohol group at position 6 of the glucose molecule readily reacts with the terminal phosphate group of ATP, forming glucose 6-phosphate and ADP. For convenience, the phosphoryl group (PO32-) is represented by Ⓟ. Because the decrease in free energy is so large, this reaction is virtually irreversible under physiological conditions.
】 the alcohol group at position 6 of the glucose molecule readily reacts with the terminal phosphate group of ATP, forming glucose 6-phosphate and ADP. For convenience, the phosphoryl group (PO32-) is represented by Ⓟ. Because the decrease in free energy is so large, this reaction is virtually irreversible under physiological conditions.
 】 the alcohol group at position 6 of the glucose molecule readily reacts with the terminal phosphate group of ATP, forming glucose 6-phosphate and ADP. For convenience, the phosphoryl group (PO32-) is represented by Ⓟ. Because the decrease in free energy is so large, this reaction is virtually irreversible under physiological conditions.
】 the alcohol group at position 6 of the glucose molecule readily reacts with the terminal phosphate group of ATP, forming glucose 6-phosphate and ADP. For convenience, the phosphoryl group (PO32-) is represented by Ⓟ. Because the decrease in free energy is so large, this reaction is virtually irreversible under physiological conditions.
In animals, this phosphorylation of glucose, which yields glucose 6-phosphate, is catalyzed by two different enzymes. In most cells a hexokinase with a high affinity for glucose—i.e., only small amounts of glucose are necessary for enzymatic activity—effects the reaction. In addition, the liver contains a glucokinase, which requires a much greater concentration of glucose before it reacts. Glucokinase functions only in emergencies, when the concentration of glucose in the blood rises to abnormally high levels.
Certain facultative anaerobic bacteria also contain hexokinases but apparently do not use them to phosphorylate glucose. In such cells, external glucose can be utilized only if it is first phosphorylated to glucose 6-phosphate via a system linked to the cell membrane that involves a compound called phosphoenolpyruvate (formed in step 【9-->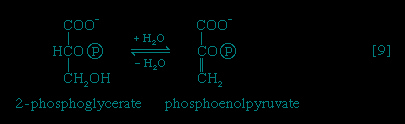 】 of glycolysis), which serves as an obligatory donor of the phosphate group; i.e., ATP cannot serve as the phosphate donor in the reaction.
】 of glycolysis), which serves as an obligatory donor of the phosphate group; i.e., ATP cannot serve as the phosphate donor in the reaction.
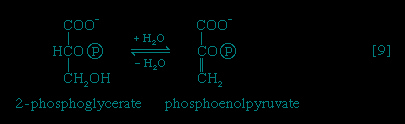 】 of glycolysis), which serves as an obligatory donor of the phosphate group; i.e., ATP cannot serve as the phosphate donor in the reaction.
】 of glycolysis), which serves as an obligatory donor of the phosphate group; i.e., ATP cannot serve as the phosphate donor in the reaction.The reaction in which glucose 6-phosphate is changed to fructose 6-phosphate is catalyzed by phosphoglucoisomerase 【2-->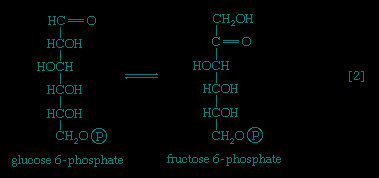 】. In the reaction, a secondary alcohol group (−C∣HOH) at the second carbon atom is oxidized to a keto-group (i.e., −C∣=O), and the aldehyde group (−CHO) at the first carbon atom is reduced to a primary alcohol group (−CH2OH). Reaction 【2-->
】. In the reaction, a secondary alcohol group (−C∣HOH) at the second carbon atom is oxidized to a keto-group (i.e., −C∣=O), and the aldehyde group (−CHO) at the first carbon atom is reduced to a primary alcohol group (−CH2OH). Reaction 【2--> 】 is readily reversible, as is indicated by the double arrows.
】 is readily reversible, as is indicated by the double arrows.
 】. In the reaction, a secondary alcohol group (−C∣HOH) at the second carbon atom is oxidized to a keto-group (i.e., −C∣=O), and the aldehyde group (−CHO) at the first carbon atom is reduced to a primary alcohol group (−CH2OH). Reaction 【2-->
】. In the reaction, a secondary alcohol group (−C∣HOH) at the second carbon atom is oxidized to a keto-group (i.e., −C∣=O), and the aldehyde group (−CHO) at the first carbon atom is reduced to a primary alcohol group (−CH2OH). Reaction 【2--> 】 is readily reversible, as is indicated by the double arrows.
】 is readily reversible, as is indicated by the double arrows.
The formation of the alcohol group at the first carbon atom permits the repetition of the reaction effected in step 【1--> 】; that is, a second molecule of ATP is invested. The product is fructose 1,6-diphosphate 【3-->
】; that is, a second molecule of ATP is invested. The product is fructose 1,6-diphosphate 【3--> 】. Again, as in the hexokinase reaction, the decrease in free energy of the reaction, which is catalyzed by phosphofructokinase, is sufficiently large to make this reaction virtually irreversible under physiological conditions; ADP is also a product.
】. Again, as in the hexokinase reaction, the decrease in free energy of the reaction, which is catalyzed by phosphofructokinase, is sufficiently large to make this reaction virtually irreversible under physiological conditions; ADP is also a product.
 】; that is, a second molecule of ATP is invested. The product is fructose 1,6-diphosphate 【3-->
】; that is, a second molecule of ATP is invested. The product is fructose 1,6-diphosphate 【3--> 】. Again, as in the hexokinase reaction, the decrease in free energy of the reaction, which is catalyzed by phosphofructokinase, is sufficiently large to make this reaction virtually irreversible under physiological conditions; ADP is also a product.
】. Again, as in the hexokinase reaction, the decrease in free energy of the reaction, which is catalyzed by phosphofructokinase, is sufficiently large to make this reaction virtually irreversible under physiological conditions; ADP is also a product.
The first three steps of glycolysis have thus transformed an asymmetrical sugar molecule, glucose, into a symmetrical form, fructose 1,6-diphosphate, containing a phosphoryl group at each end; the molecule next is split into two smaller fragments that are interconvertible. This elegant simplification is achieved via steps 【4-->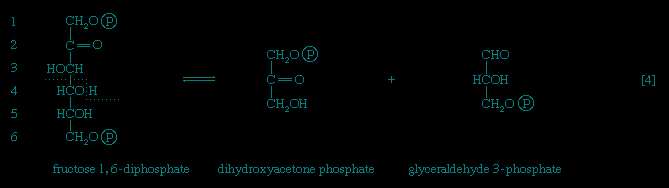 】 and 【5-->
】 and 【5-->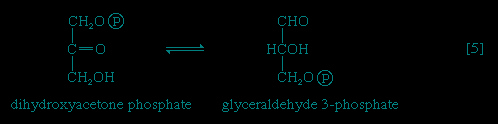 】, which are described below.
】, which are described below.
 】 and 【5-->
】 and 【5--> 】, which are described below.
】, which are described below.
The aldolase reaction
In 【4--> 】, an enzyme catalyzes the breaking apart of the six-carbon sugar fructose 1,6-diphosphate into two three-carbon fragments. The molecule is split between carbons 3 and 4. Reversal of this cleavage—i.e., the formation of a six-carbon compound from two three-carbon compounds—is possible. Because the reverse reaction is an aldol condensation—i.e., an aldehyde (glyceraldehyde 3-phosphate) combines with a ketone (dihydroxyacetone phosphate)—the enzyme is commonly called aldolase. The two three-carbon fragments produced in step 【4-->
】, an enzyme catalyzes the breaking apart of the six-carbon sugar fructose 1,6-diphosphate into two three-carbon fragments. The molecule is split between carbons 3 and 4. Reversal of this cleavage—i.e., the formation of a six-carbon compound from two three-carbon compounds—is possible. Because the reverse reaction is an aldol condensation—i.e., an aldehyde (glyceraldehyde 3-phosphate) combines with a ketone (dihydroxyacetone phosphate)—the enzyme is commonly called aldolase. The two three-carbon fragments produced in step 【4--> 】, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, are also called triose phosphates. They are readily converted to each other by a process 【5-->
】, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, are also called triose phosphates. They are readily converted to each other by a process 【5--> 】 analogous to that in step 【2-->
】 analogous to that in step 【2--> 】. The enzyme that catalyzes the interconversion 【5-->
】. The enzyme that catalyzes the interconversion 【5--> 】 is triose phosphate isomerase, a different enzyme than that catalyzing step 【2-->
】 is triose phosphate isomerase, a different enzyme than that catalyzing step 【2--> 】.
】.
 】, an enzyme catalyzes the breaking apart of the six-carbon sugar fructose 1,6-diphosphate into two three-carbon fragments. The molecule is split between carbons 3 and 4. Reversal of this cleavage—i.e., the formation of a six-carbon compound from two three-carbon compounds—is possible. Because the reverse reaction is an aldol condensation—i.e., an aldehyde (glyceraldehyde 3-phosphate) combines with a ketone (dihydroxyacetone phosphate)—the enzyme is commonly called aldolase. The two three-carbon fragments produced in step 【4-->
】, an enzyme catalyzes the breaking apart of the six-carbon sugar fructose 1,6-diphosphate into two three-carbon fragments. The molecule is split between carbons 3 and 4. Reversal of this cleavage—i.e., the formation of a six-carbon compound from two three-carbon compounds—is possible. Because the reverse reaction is an aldol condensation—i.e., an aldehyde (glyceraldehyde 3-phosphate) combines with a ketone (dihydroxyacetone phosphate)—the enzyme is commonly called aldolase. The two three-carbon fragments produced in step 【4--> 】, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, are also called triose phosphates. They are readily converted to each other by a process 【5-->
】, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, are also called triose phosphates. They are readily converted to each other by a process 【5--> 】 analogous to that in step 【2-->
】 analogous to that in step 【2--> 】. The enzyme that catalyzes the interconversion 【5-->
】. The enzyme that catalyzes the interconversion 【5--> 】 is triose phosphate isomerase, a different enzyme than that catalyzing step 【2-->
】 is triose phosphate isomerase, a different enzyme than that catalyzing step 【2--> 】.
】.
The formation of ATP
The second stage of glucose catabolism comprises reactions 【6】 through 【10】, in which a net gain of ATP is achieved through the oxidation of one of the triose phosphate compounds formed in step 【5--> 】. One molecule of glucose forms two molecules of the triose phosphate; both three-carbon fragments follow the same pathway, and steps 【6】 through 【10】 must occur twice to complete the glucose breakdown.
】. One molecule of glucose forms two molecules of the triose phosphate; both three-carbon fragments follow the same pathway, and steps 【6】 through 【10】 must occur twice to complete the glucose breakdown.
 】. One molecule of glucose forms two molecules of the triose phosphate; both three-carbon fragments follow the same pathway, and steps 【6】 through 【10】 must occur twice to complete the glucose breakdown.
】. One molecule of glucose forms two molecules of the triose phosphate; both three-carbon fragments follow the same pathway, and steps 【6】 through 【10】 must occur twice to complete the glucose breakdown.
Step 【6--> 】, in which glyceraldehyde 3-phosphate is oxidized, is one of the most important reactions in glycolysis. It is during this step that the energy liberated during oxidation of the aldehyde group (−CHO) is conserved in the form of a high-energy phosphate compound; namely, as 1,3-diphosphoglycerate, an anhydride of a carboxylic acid and phosphoric acid. The hydrogen atoms or electrons removed from the aldehyde group during its oxidation are accepted by a coenzyme (so called because it functions in conjunction with an enzyme) involved in hydrogen or electron transfer; the coenzyme, nicotinamide adenine dinucleotide (NAD+), is reduced to form NADH + H+ in the process. The NAD+ thus reduced is bound to the enzyme glyceraldehyde 3-phosphate dehydrogenase, catalyzing the overall reaction, step 【6-->
】, in which glyceraldehyde 3-phosphate is oxidized, is one of the most important reactions in glycolysis. It is during this step that the energy liberated during oxidation of the aldehyde group (−CHO) is conserved in the form of a high-energy phosphate compound; namely, as 1,3-diphosphoglycerate, an anhydride of a carboxylic acid and phosphoric acid. The hydrogen atoms or electrons removed from the aldehyde group during its oxidation are accepted by a coenzyme (so called because it functions in conjunction with an enzyme) involved in hydrogen or electron transfer; the coenzyme, nicotinamide adenine dinucleotide (NAD+), is reduced to form NADH + H+ in the process. The NAD+ thus reduced is bound to the enzyme glyceraldehyde 3-phosphate dehydrogenase, catalyzing the overall reaction, step 【6--> 】.
】.
 】, in which glyceraldehyde 3-phosphate is oxidized, is one of the most important reactions in glycolysis. It is during this step that the energy liberated during oxidation of the aldehyde group (−CHO) is conserved in the form of a high-energy phosphate compound; namely, as 1,3-diphosphoglycerate, an anhydride of a carboxylic acid and phosphoric acid. The hydrogen atoms or electrons removed from the aldehyde group during its oxidation are accepted by a coenzyme (so called because it functions in conjunction with an enzyme) involved in hydrogen or electron transfer; the coenzyme, nicotinamide adenine dinucleotide (NAD+), is reduced to form NADH + H+ in the process. The NAD+ thus reduced is bound to the enzyme glyceraldehyde 3-phosphate dehydrogenase, catalyzing the overall reaction, step 【6-->
】, in which glyceraldehyde 3-phosphate is oxidized, is one of the most important reactions in glycolysis. It is during this step that the energy liberated during oxidation of the aldehyde group (−CHO) is conserved in the form of a high-energy phosphate compound; namely, as 1,3-diphosphoglycerate, an anhydride of a carboxylic acid and phosphoric acid. The hydrogen atoms or electrons removed from the aldehyde group during its oxidation are accepted by a coenzyme (so called because it functions in conjunction with an enzyme) involved in hydrogen or electron transfer; the coenzyme, nicotinamide adenine dinucleotide (NAD+), is reduced to form NADH + H+ in the process. The NAD+ thus reduced is bound to the enzyme glyceraldehyde 3-phosphate dehydrogenase, catalyzing the overall reaction, step 【6--> 】.
】.The 1,3-diphosphoglycerate produced in step 【6--> 】 reacts with ADP in a reaction catalyzed by phosphoglycerate kinase, with the result that one of the two phosphoryl groups is transferred to ADP to form ATP and 3-phosphoglycerate. This reaction 【7-->
】 reacts with ADP in a reaction catalyzed by phosphoglycerate kinase, with the result that one of the two phosphoryl groups is transferred to ADP to form ATP and 3-phosphoglycerate. This reaction 【7-->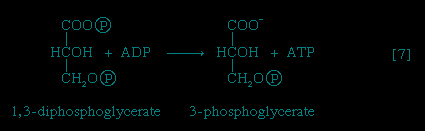 】 is highly exergonic (i.e., it proceeds with a loss of free energy); as a result, the oxidation of glyceraldehyde 3-phosphate, step 【6-->
】 is highly exergonic (i.e., it proceeds with a loss of free energy); as a result, the oxidation of glyceraldehyde 3-phosphate, step 【6--> 】, is irreversible. In summary, the energy liberated during oxidation of an aldehyde group (−CHO in glyceraldehyde 3-phosphate) to a carboxylic acid group (−COO- in 3-phosphoglycerate) is conserved as the phosphate bond energy in ATP during steps 【6-->
】, is irreversible. In summary, the energy liberated during oxidation of an aldehyde group (−CHO in glyceraldehyde 3-phosphate) to a carboxylic acid group (−COO- in 3-phosphoglycerate) is conserved as the phosphate bond energy in ATP during steps 【6--> 】 and 【7-->
】 and 【7--> 】. This step occurs twice for each molecule of glucose; thus the initial investment of ATP in steps 【1-->
】. This step occurs twice for each molecule of glucose; thus the initial investment of ATP in steps 【1--> 】 and 【3-->
】 and 【3--> 】 is recovered.
】 is recovered.
 】 reacts with ADP in a reaction catalyzed by phosphoglycerate kinase, with the result that one of the two phosphoryl groups is transferred to ADP to form ATP and 3-phosphoglycerate. This reaction 【7-->
】 reacts with ADP in a reaction catalyzed by phosphoglycerate kinase, with the result that one of the two phosphoryl groups is transferred to ADP to form ATP and 3-phosphoglycerate. This reaction 【7--> 】 is highly exergonic (i.e., it proceeds with a loss of free energy); as a result, the oxidation of glyceraldehyde 3-phosphate, step 【6-->
】 is highly exergonic (i.e., it proceeds with a loss of free energy); as a result, the oxidation of glyceraldehyde 3-phosphate, step 【6--> 】, is irreversible. In summary, the energy liberated during oxidation of an aldehyde group (−CHO in glyceraldehyde 3-phosphate) to a carboxylic acid group (−COO- in 3-phosphoglycerate) is conserved as the phosphate bond energy in ATP during steps 【6-->
】, is irreversible. In summary, the energy liberated during oxidation of an aldehyde group (−CHO in glyceraldehyde 3-phosphate) to a carboxylic acid group (−COO- in 3-phosphoglycerate) is conserved as the phosphate bond energy in ATP during steps 【6--> 】 and 【7-->
】 and 【7--> 】. This step occurs twice for each molecule of glucose; thus the initial investment of ATP in steps 【1-->
】. This step occurs twice for each molecule of glucose; thus the initial investment of ATP in steps 【1--> 】 and 【3-->
】 and 【3--> 】 is recovered.
】 is recovered.
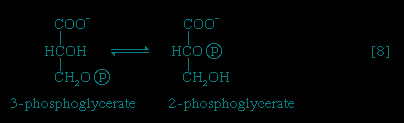
The 3-phosphoglycerate in step 【7--> 】 now forms 2-phosphoglycerate, in a reaction catalyzed by phosphoglyceromutase 【8-->
】 now forms 2-phosphoglycerate, in a reaction catalyzed by phosphoglyceromutase 【8-->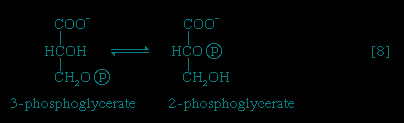 】. During step 【9-->
】. During step 【9-->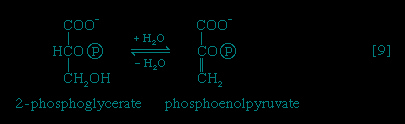 】 the enzyme enolase reacts with 2-phosphoglycerate to form phosphoenolpyruvate (PEP), water being lost from 2-phosphoglycerate in the process. Phosphoenolpyruvate acts as the second source of ATP in glycolysis. The transfer of the phosphate group from PEP to ADP, catalyzed by pyruvate kinase 【10-->
】 the enzyme enolase reacts with 2-phosphoglycerate to form phosphoenolpyruvate (PEP), water being lost from 2-phosphoglycerate in the process. Phosphoenolpyruvate acts as the second source of ATP in glycolysis. The transfer of the phosphate group from PEP to ADP, catalyzed by pyruvate kinase 【10-->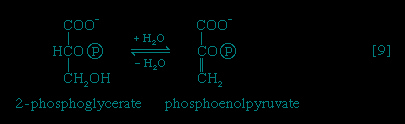 】, is also highly exergonic and is thus virtually irreversible under physiological conditions.
】, is also highly exergonic and is thus virtually irreversible under physiological conditions.
 】 now forms 2-phosphoglycerate, in a reaction catalyzed by phosphoglyceromutase 【8-->
】 now forms 2-phosphoglycerate, in a reaction catalyzed by phosphoglyceromutase 【8-->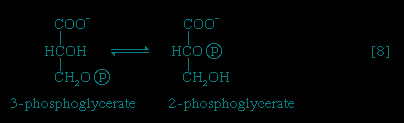 】. During step 【9-->
】. During step 【9-->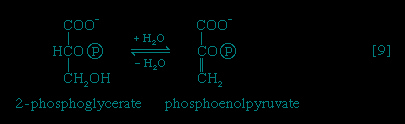 】 the enzyme enolase reacts with 2-phosphoglycerate to form phosphoenolpyruvate (PEP), water being lost from 2-phosphoglycerate in the process. Phosphoenolpyruvate acts as the second source of ATP in glycolysis. The transfer of the phosphate group from PEP to ADP, catalyzed by pyruvate kinase 【10-->
】 the enzyme enolase reacts with 2-phosphoglycerate to form phosphoenolpyruvate (PEP), water being lost from 2-phosphoglycerate in the process. Phosphoenolpyruvate acts as the second source of ATP in glycolysis. The transfer of the phosphate group from PEP to ADP, catalyzed by pyruvate kinase 【10-->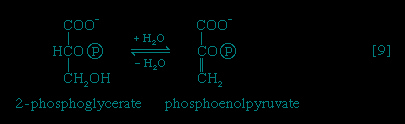 】, is also highly exergonic and is thus virtually irreversible under physiological conditions.
】, is also highly exergonic and is thus virtually irreversible under physiological conditions.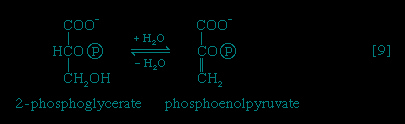
Reaction 【10--> 】 occurs twice for each molecule of glucose entering the glycolytic sequence; thus the net yield is two molecules of ATP for each six-carbon sugar. No further molecules of glucose can enter the glycolytic pathway, however, until the NADH + H+ produced in step 【6-->
】 occurs twice for each molecule of glucose entering the glycolytic sequence; thus the net yield is two molecules of ATP for each six-carbon sugar. No further molecules of glucose can enter the glycolytic pathway, however, until the NADH + H+ produced in step 【6--> 】 is reoxidized to NAD+. In anaerobic systems this means that electrons must be transferred from (NADH + H+) to some
】 is reoxidized to NAD+. In anaerobic systems this means that electrons must be transferred from (NADH + H+) to some
 】 occurs twice for each molecule of glucose entering the glycolytic sequence; thus the net yield is two molecules of ATP for each six-carbon sugar. No further molecules of glucose can enter the glycolytic pathway, however, until the NADH + H+ produced in step 【6-->
】 occurs twice for each molecule of glucose entering the glycolytic sequence; thus the net yield is two molecules of ATP for each six-carbon sugar. No further molecules of glucose can enter the glycolytic pathway, however, until the NADH + H+ produced in step 【6--> 】 is reoxidized to NAD+. In anaerobic systems this means that electrons must be transferred from (NADH + H+) to some
】 is reoxidized to NAD+. In anaerobic systems this means that electrons must be transferred from (NADH + H+) to some
organic acceptor molecule, which thus is reduced in the process. Such an acceptor molecule could be the pyruvate formed in reaction 【10--> 】. In certain bacteria (e.g., so-called lactic acid bacteria) or in muscle cells functioning vigorously in the absence of adequate supplies of oxygen, pyruvate is reduced to lactate via a reaction catalyzed by lactate dehydrogenase (reaction 【11a-->
】. In certain bacteria (e.g., so-called lactic acid bacteria) or in muscle cells functioning vigorously in the absence of adequate supplies of oxygen, pyruvate is reduced to lactate via a reaction catalyzed by lactate dehydrogenase (reaction 【11a-->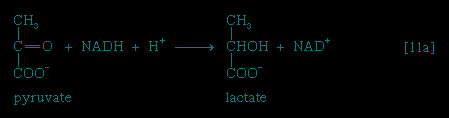 】); i.e., NADH gives up its hydrogen
】); i.e., NADH gives up its hydrogen
 】. In certain bacteria (e.g., so-called lactic acid bacteria) or in muscle cells functioning vigorously in the absence of adequate supplies of oxygen, pyruvate is reduced to lactate via a reaction catalyzed by lactate dehydrogenase (reaction 【11a-->
】. In certain bacteria (e.g., so-called lactic acid bacteria) or in muscle cells functioning vigorously in the absence of adequate supplies of oxygen, pyruvate is reduced to lactate via a reaction catalyzed by lactate dehydrogenase (reaction 【11a--> 】); i.e., NADH gives up its hydrogen
】); i.e., NADH gives up its hydrogen
atoms or electrons to pyruvate, and lactate and NAD+ are formed. Alternatively, in organisms such as brewers' yeast, pyruvate is first decarboxylated to form acetaldehyde and carbon dioxide in a reaction catalyzed by pyruvate decarboxylase 【11b-->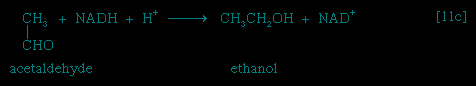 】; acetaldehyde then is reduced
】; acetaldehyde then is reduced
 】; acetaldehyde then is reduced
】; acetaldehyde then is reduced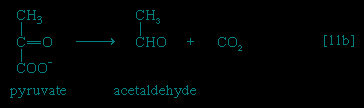
(by NADH + H+) in a reaction catalyzed by alcohol dehydrogenase 【11c--> 】, yielding ethanol and oxidized coenzyme (NAD+).
】, yielding ethanol and oxidized coenzyme (NAD+).
 】, yielding ethanol and oxidized coenzyme (NAD+).
】, yielding ethanol and oxidized coenzyme (NAD+).
Many variations of reactions 【11a--> , b-->
, b-->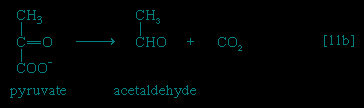 , and c-->
, and c--> 】 occur in nature. In the heterolactic (mixed lactic acid) fermentations carried out by some microorganisms, a mixture of reactions 【11a-->
】 occur in nature. In the heterolactic (mixed lactic acid) fermentations carried out by some microorganisms, a mixture of reactions 【11a--> , b-->
, b-->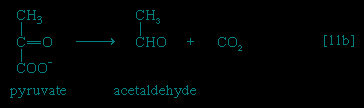 , and c-->
, and c--> 】 regenerates NAD+ and results in the production, for each molecule of glucose fermented, of a molecule each of lactate, ethanol, and carbon dioxide. In other types of fermentation, the end products may be derivatives of acids such as propionic, butyric, acetic, and succinic; decarboxylated materials derived from them (e.g., acetone); or compounds such as glycerol.
】 regenerates NAD+ and results in the production, for each molecule of glucose fermented, of a molecule each of lactate, ethanol, and carbon dioxide. In other types of fermentation, the end products may be derivatives of acids such as propionic, butyric, acetic, and succinic; decarboxylated materials derived from them (e.g., acetone); or compounds such as glycerol.
 , b-->
, b-->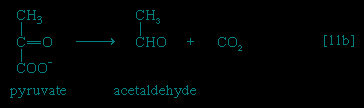 , and c-->
, and c--> 】 occur in nature. In the heterolactic (mixed lactic acid) fermentations carried out by some microorganisms, a mixture of reactions 【11a-->
】 occur in nature. In the heterolactic (mixed lactic acid) fermentations carried out by some microorganisms, a mixture of reactions 【11a--> , b-->
, b-->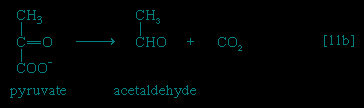 , and c-->
, and c--> 】 regenerates NAD+ and results in the production, for each molecule of glucose fermented, of a molecule each of lactate, ethanol, and carbon dioxide. In other types of fermentation, the end products may be derivatives of acids such as propionic, butyric, acetic, and succinic; decarboxylated materials derived from them (e.g., acetone); or compounds such as glycerol.
】 regenerates NAD+ and results in the production, for each molecule of glucose fermented, of a molecule each of lactate, ethanol, and carbon dioxide. In other types of fermentation, the end products may be derivatives of acids such as propionic, butyric, acetic, and succinic; decarboxylated materials derived from them (e.g., acetone); or compounds such as glycerol.The phosphogluconate pathway
Many cells possess, in addition to all or part of the glycolytic pathway that comprises reactions 【1--> ,2-->
,2--> ,3-->
,3--> ,4-->
,4--> ,5-->
,5--> ,6-->
,6--> ,7-->
,7--> ,8-->
,8-->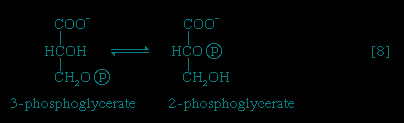 ,9-->
,9-->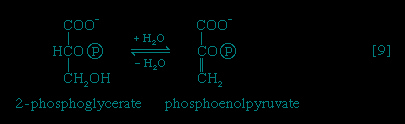 ,10-->
,10--> ,11-->
,11--> 】, other pathways of glucose catabolism that involve, as the first unique step, the oxidation of glucose 6-phosphate 【12-->
】, other pathways of glucose catabolism that involve, as the first unique step, the oxidation of glucose 6-phosphate 【12-->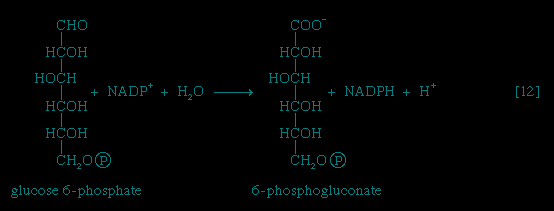 】 instead of the formation of fructose 6-phosphate 【2-->
】 instead of the formation of fructose 6-phosphate 【2--> 】. This is the phosphogluconate pathway, or pentose phosphate cycle. During reaction 【12-->
】. This is the phosphogluconate pathway, or pentose phosphate cycle. During reaction 【12--> 】, hydrogen atoms or electrons are removed from the carbon atom at position 1 of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphate dehydrogenase. The product of the reaction is 6-phosphogluconate.
】, hydrogen atoms or electrons are removed from the carbon atom at position 1 of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphate dehydrogenase. The product of the reaction is 6-phosphogluconate.
 ,2-->
,2--> ,3-->
,3--> ,4-->
,4--> ,5-->
,5--> ,6-->
,6--> ,7-->
,7--> ,8-->
,8-->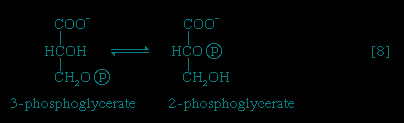 ,9-->
,9-->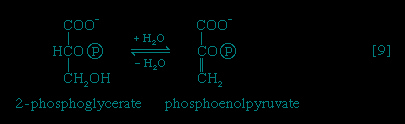 ,10-->
,10--> ,11-->
,11--> 】, other pathways of glucose catabolism that involve, as the first unique step, the oxidation of glucose 6-phosphate 【12-->
】, other pathways of glucose catabolism that involve, as the first unique step, the oxidation of glucose 6-phosphate 【12--> 】 instead of the formation of fructose 6-phosphate 【2-->
】 instead of the formation of fructose 6-phosphate 【2--> 】. This is the phosphogluconate pathway, or pentose phosphate cycle. During reaction 【12-->
】. This is the phosphogluconate pathway, or pentose phosphate cycle. During reaction 【12--> 】, hydrogen atoms or electrons are removed from the carbon atom at position 1 of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphate dehydrogenase. The product of the reaction is 6-phosphogluconate.
】, hydrogen atoms or electrons are removed from the carbon atom at position 1 of glucose 6-phosphate in a reaction catalyzed by glucose 6-phosphate dehydrogenase. The product of the reaction is 6-phosphogluconate.
The reducing equivalents (hydrogen atoms or electrons) are accepted by nicotine adenine dinucleotide phosphate (NADP+), a coenzyme similar to but not identical with NAD+. A second molecule of NADP+ is reduced as 6-phosphogluconate is further oxidized; the reaction is catalyzed by 6-phosphogluconate dehydrogenase 【13--> 】. The products of the reaction also include ribulose 5-phosphate and carbon dioxide. (The numbers at the carbon atoms in step 【13-->
】. The products of the reaction also include ribulose 5-phosphate and carbon dioxide. (The numbers at the carbon atoms in step 【13-->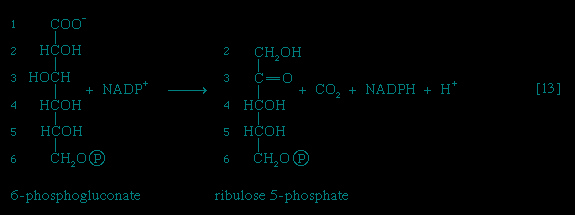 】 indicate that carbon 1 of 6-phosphogluconate forms carbon dioxide.)
】 indicate that carbon 1 of 6-phosphogluconate forms carbon dioxide.)
 】. The products of the reaction also include ribulose 5-phosphate and carbon dioxide. (The numbers at the carbon atoms in step 【13-->
】. The products of the reaction also include ribulose 5-phosphate and carbon dioxide. (The numbers at the carbon atoms in step 【13--> 】 indicate that carbon 1 of 6-phosphogluconate forms carbon dioxide.)
】 indicate that carbon 1 of 6-phosphogluconate forms carbon dioxide.)
 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> Ribulose 5-phosphate can undergo a series of reactions in which two-carbon and three-carbon fragments are interchanged between a number of sugar phosphates; this sequence of events can lead to the formation of two molecules of fructose 6-phosphate and one of glyceraldehyde 3-phosphate from three molecules of ribulose 5-phosphate (i.e., the conversion of three molecules with five carbons to two with six and one with three). Although the cycle, which is outlined in Figure 4-->
Ribulose 5-phosphate can undergo a series of reactions in which two-carbon and three-carbon fragments are interchanged between a number of sugar phosphates; this sequence of events can lead to the formation of two molecules of fructose 6-phosphate and one of glyceraldehyde 3-phosphate from three molecules of ribulose 5-phosphate (i.e., the conversion of three molecules with five carbons to two with six and one with three). Although the cycle, which is outlined in Figure 4--> , is the main pathway in microorganisms for fragmentation of pentose sugars, it is not of major importance as a route for the oxidation of glucose. Its primary purpose in most cells is to generate reducing power in the cytoplasm, in the form of reduced NADP+. This function is especially prominent in tissues—such as the liver, mammary gland, fat tissue, and the cortex (outer region) of the adrenal gland—that actively carry out the biosynthesis of fatty acids and other fatty substances (e.g., steroids). A second function of reactions 【12-->
, is the main pathway in microorganisms for fragmentation of pentose sugars, it is not of major importance as a route for the oxidation of glucose. Its primary purpose in most cells is to generate reducing power in the cytoplasm, in the form of reduced NADP+. This function is especially prominent in tissues—such as the liver, mammary gland, fat tissue, and the cortex (outer region) of the adrenal gland—that actively carry out the biosynthesis of fatty acids and other fatty substances (e.g., steroids). A second function of reactions 【12--> 】 and 【13-->
】 and 【13--> 】 is to generate from glucose 6-phosphate the pentoses that are used in the synthesis of nucleic acids (see below The biosynthesis of cell components (metabolism)).
】 is to generate from glucose 6-phosphate the pentoses that are used in the synthesis of nucleic acids (see below The biosynthesis of cell components (metabolism)).In photosynthetic organisms, some of the reactions of the phosphogluconate pathway are part of the major route for the formation of sugars from carbon dioxide; in this case, the reactions occur in a direction opposite to that in which they occur in nonphotosynthetic tissues (see photosynthesis).
A different route for the catabolism of glucose also involves 6-phosphogluconate; it is of considerable importance in microorganisms lacking some of the enzymes necessary for glycolysis. In this route, 6-phosphogluconate (derived from glucose via steps 【1--> 】 and 【12-->
】 and 【12--> 】) is not oxidized to ribulose 5-phosphate via reaction 【13-->
】) is not oxidized to ribulose 5-phosphate via reaction 【13--> 】 but, in an enzyme-catalyzed reaction 【14-->
】 but, in an enzyme-catalyzed reaction 【14-->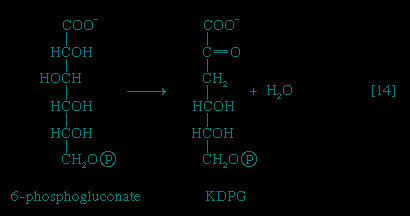 】, loses water, forming the compound 2-keto-3-deoxy-6-phosphogluconate (KDPG).
】, loses water, forming the compound 2-keto-3-deoxy-6-phosphogluconate (KDPG).
 】 and 【12-->
】 and 【12--> 】) is not oxidized to ribulose 5-phosphate via reaction 【13-->
】) is not oxidized to ribulose 5-phosphate via reaction 【13--> 】 but, in an enzyme-catalyzed reaction 【14-->
】 but, in an enzyme-catalyzed reaction 【14--> 】, loses water, forming the compound 2-keto-3-deoxy-6-phosphogluconate (KDPG).
】, loses water, forming the compound 2-keto-3-deoxy-6-phosphogluconate (KDPG).
This is then split into pyruvate and glyceraldehyde-3-phosphate 【15-->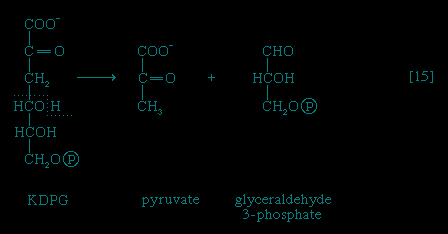 】, both of which are intermediates of the glycolytic pathway.
】, both of which are intermediates of the glycolytic pathway.
 】, both of which are intermediates of the glycolytic pathway.
】, both of which are intermediates of the glycolytic pathway.
The catabolism of sugars other than glucose
Release of glucose from glycogen
The main storage carbohydrate of animal cells is glycogen, in which chains of glucose molecules—linked end-to-end, the C1 position of one glucose being linked to the C4 position of the adjacent one—are joined to each other by occasional linkages between a carbon at position 1 on one glucose and a carbon at position 6 on another. Two enzymes cooperate in releasing glucose molecules from glycogen. Glycogen phosphorylase catalyzes the splitting of the 1,4-bonds by adding the elements of phosphoric acid at the point shown by the broken arrow in 【16-->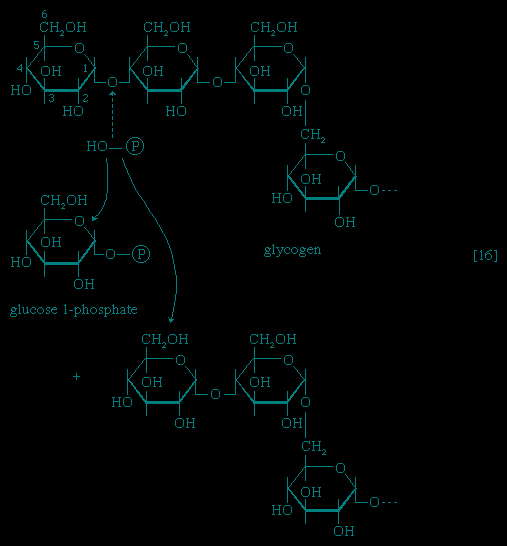 】, rather than water, as in the digestive hydrolysis of polysaccharides such as glycogen and starch. The products of 【16-->
】, rather than water, as in the digestive hydrolysis of polysaccharides such as glycogen and starch. The products of 【16--> 】 are glucose 1-phosphate and chains of sugar molecules shortened by one unit; the chains are degraded further by repetition of step 【16-->
】 are glucose 1-phosphate and chains of sugar molecules shortened by one unit; the chains are degraded further by repetition of step 【16--> 】. When a bridge linking two chains, at C1 and C6 carbon atoms of adjacent glucose units, is reached, it is hydrolyzed in a reaction involving the enzyme α (1 → 6) glucosidase. After the two chains are separated, reaction 【16-->
】. When a bridge linking two chains, at C1 and C6 carbon atoms of adjacent glucose units, is reached, it is hydrolyzed in a reaction involving the enzyme α (1 → 6) glucosidase. After the two chains are separated, reaction 【16--> 】 can occur again. The glucose 1-phosphate thus formed from glycogen or, in plants, from starch, is converted to glucose 6-phosphate by phosphoglucomutase 【78-->
】 can occur again. The glucose 1-phosphate thus formed from glycogen or, in plants, from starch, is converted to glucose 6-phosphate by phosphoglucomutase 【78--> 】, which catalyzes a reaction very similar to that effected in step 【8-->
】, which catalyzes a reaction very similar to that effected in step 【8-->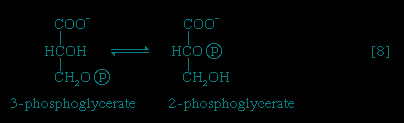 】 of glycolysis; glucose 6-phosphate can then undergo further catabolism via glycolysis 【2-->
】 of glycolysis; glucose 6-phosphate can then undergo further catabolism via glycolysis 【2--> ,3-->
,3--> ,4-->
,4--> ,5-->
,5--> ,6-->
,6--> ,7-->
,7--> ,8-->
,8-->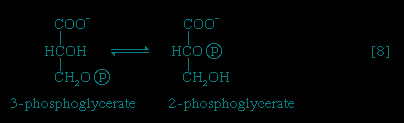 ,9-->
,9-->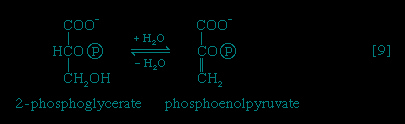 ,10-->
,10--> 】 or via either of the routes involving formation of 6-phosphogluconate 【12-->
】 or via either of the routes involving formation of 6-phosphogluconate 【12--> 】.
】.
 】, rather than water, as in the digestive hydrolysis of polysaccharides such as glycogen and starch. The products of 【16-->
】, rather than water, as in the digestive hydrolysis of polysaccharides such as glycogen and starch. The products of 【16--> 】 are glucose 1-phosphate and chains of sugar molecules shortened by one unit; the chains are degraded further by repetition of step 【16-->
】 are glucose 1-phosphate and chains of sugar molecules shortened by one unit; the chains are degraded further by repetition of step 【16--> 】. When a bridge linking two chains, at C1 and C6 carbon atoms of adjacent glucose units, is reached, it is hydrolyzed in a reaction involving the enzyme α (1 → 6) glucosidase. After the two chains are separated, reaction 【16-->
】. When a bridge linking two chains, at C1 and C6 carbon atoms of adjacent glucose units, is reached, it is hydrolyzed in a reaction involving the enzyme α (1 → 6) glucosidase. After the two chains are separated, reaction 【16--> 】 can occur again. The glucose 1-phosphate thus formed from glycogen or, in plants, from starch, is converted to glucose 6-phosphate by phosphoglucomutase 【78-->
】 can occur again. The glucose 1-phosphate thus formed from glycogen or, in plants, from starch, is converted to glucose 6-phosphate by phosphoglucomutase 【78--> 】, which catalyzes a reaction very similar to that effected in step 【8-->
】, which catalyzes a reaction very similar to that effected in step 【8-->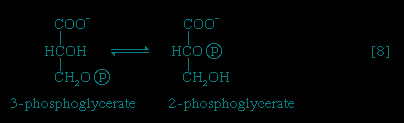 】 of glycolysis; glucose 6-phosphate can then undergo further catabolism via glycolysis 【2-->
】 of glycolysis; glucose 6-phosphate can then undergo further catabolism via glycolysis 【2--> ,3-->
,3--> ,4-->
,4--> ,5-->
,5--> ,6-->
,6--> ,7-->
,7--> ,8-->
,8-->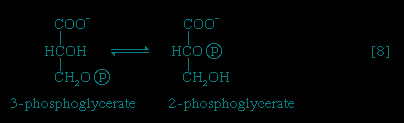 ,9-->
,9-->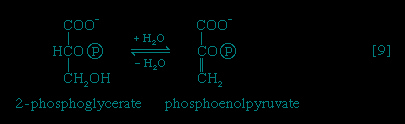 ,10-->
,10--> 】 or via either of the routes involving formation of 6-phosphogluconate 【12-->
】 or via either of the routes involving formation of 6-phosphogluconate 【12--> 】.
】.
Fragmentation of other sugars
Other sugars encountered in the diet are likewise transformed to products that are intermediates of central metabolic pathways. lactose, or milk sugar, is composed of one molecule of galactose linked to one molecule of glucose. Sucrose, the common sugar of cane or beet, is made up of glucose linked to fructose. Both sucrose and lactose are hydrolyzed to glucose and fructose or galactose, respectively. Glucose is utilized as already described, but special reactions must occur before the other sugars can enter the catabolic routes. Galactose, for example, is phosphorylated in a manner analogous to step 【1--> 】 of glycolysis. The reaction, catalyzed by a galactokinase, results in the formation of galactose 1-phosphate; this product is transformed to glucose 1-phosphate by a sequence of reactions requiring as a coenzyme uridine triphosphate (UTP). Fructose may also be phosphorylated in animal cells through the action of hexokinase 【1-->
】 of glycolysis. The reaction, catalyzed by a galactokinase, results in the formation of galactose 1-phosphate; this product is transformed to glucose 1-phosphate by a sequence of reactions requiring as a coenzyme uridine triphosphate (UTP). Fructose may also be phosphorylated in animal cells through the action of hexokinase 【1--> 】, in which case fructose 6-phosphate is the product, or in liver tissue via a fructokinase that gives rise to fructose 1-phosphate 【17-->
】, in which case fructose 6-phosphate is the product, or in liver tissue via a fructokinase that gives rise to fructose 1-phosphate 【17-->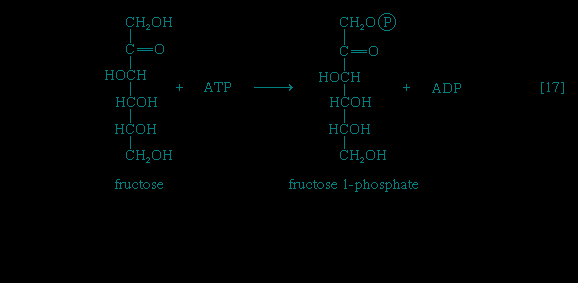 】. Adenosine triphosphate supplies the phosphate group in both cases.
】. Adenosine triphosphate supplies the phosphate group in both cases.
 】 of glycolysis. The reaction, catalyzed by a galactokinase, results in the formation of galactose 1-phosphate; this product is transformed to glucose 1-phosphate by a sequence of reactions requiring as a coenzyme uridine triphosphate (UTP). Fructose may also be phosphorylated in animal cells through the action of hexokinase 【1-->
】 of glycolysis. The reaction, catalyzed by a galactokinase, results in the formation of galactose 1-phosphate; this product is transformed to glucose 1-phosphate by a sequence of reactions requiring as a coenzyme uridine triphosphate (UTP). Fructose may also be phosphorylated in animal cells through the action of hexokinase 【1--> 】, in which case fructose 6-phosphate is the product, or in liver tissue via a fructokinase that gives rise to fructose 1-phosphate 【17-->
】, in which case fructose 6-phosphate is the product, or in liver tissue via a fructokinase that gives rise to fructose 1-phosphate 【17--> 】. Adenosine triphosphate supplies the phosphate group in both cases.
】. Adenosine triphosphate supplies the phosphate group in both cases.
Fructose 1-phosphate is also formed when facultative anaerobic microorganisms use fructose as a carbon source for growth; in this case, however, the source of the phosphate is phosphoenolpyruvate rather than ATP. Fructose 1-phosphate can be catabolized by one of two routes. In the liver, it is split by an aldolase enzyme 【18-->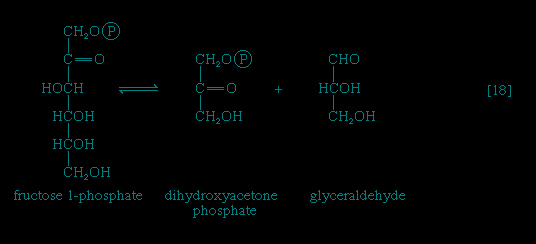 】 abundant in that tissue (but lacking in muscle); the products are dihydroxyacetone phosphate and glyceraldehyde. It will be recalled that dihydroxyacetone phosphate is an intermediate compound of glycolysis. Although glyceraldehyde is not an intermediate of glycolysis, it can be converted to one (glyceraldehyde 3-phosphate) in a reaction involving the conversion of ATP to ADP.
】 abundant in that tissue (but lacking in muscle); the products are dihydroxyacetone phosphate and glyceraldehyde. It will be recalled that dihydroxyacetone phosphate is an intermediate compound of glycolysis. Although glyceraldehyde is not an intermediate of glycolysis, it can be converted to one (glyceraldehyde 3-phosphate) in a reaction involving the conversion of ATP to ADP.
 】 abundant in that tissue (but lacking in muscle); the products are dihydroxyacetone phosphate and glyceraldehyde. It will be recalled that dihydroxyacetone phosphate is an intermediate compound of glycolysis. Although glyceraldehyde is not an intermediate of glycolysis, it can be converted to one (glyceraldehyde 3-phosphate) in a reaction involving the conversion of ATP to ADP.
】 abundant in that tissue (but lacking in muscle); the products are dihydroxyacetone phosphate and glyceraldehyde. It will be recalled that dihydroxyacetone phosphate is an intermediate compound of glycolysis. Although glyceraldehyde is not an intermediate of glycolysis, it can be converted to one (glyceraldehyde 3-phosphate) in a reaction involving the conversion of ATP to ADP.
In many organisms other than mammals, fructose 1-phosphate does not have to undergo reaction 【18--> 】 in order to enter central metabolic routes. Instead, a fructose 1-phosphate kinase, distinct from the phosphofructokinase that catalyzes step 【3-->
】 in order to enter central metabolic routes. Instead, a fructose 1-phosphate kinase, distinct from the phosphofructokinase that catalyzes step 【3--> 】 of glycolysis, effects the direct conversion of fructose 1-phosphate and ATP to fructose 1,6-diphosphate and ADP.
】 of glycolysis, effects the direct conversion of fructose 1-phosphate and ATP to fructose 1,6-diphosphate and ADP.
 】 in order to enter central metabolic routes. Instead, a fructose 1-phosphate kinase, distinct from the phosphofructokinase that catalyzes step 【3-->
】 in order to enter central metabolic routes. Instead, a fructose 1-phosphate kinase, distinct from the phosphofructokinase that catalyzes step 【3--> 】 of glycolysis, effects the direct conversion of fructose 1-phosphate and ATP to fructose 1,6-diphosphate and ADP.
】 of glycolysis, effects the direct conversion of fructose 1-phosphate and ATP to fructose 1,6-diphosphate and ADP.The catabolism of lipids (fats)
Although carbohydrates are the major fuel for most organisms, fatty acids (fatty acid) are also a very important energy source. In vertebrates at least half of the oxidative energy used by the liver, kidneys, heart muscle, and resting skeletal muscle is derived from the oxidation of fatty acids; in fasting or hibernating animals or in migrating birds, fat is virtually the sole source of energy.
Neutral fats or triglycerides (triglyceride), the major components of storage fats in plant and animal cells, consist of the alcohol glycerol linked to three molecules of fatty acids. Before a molecule of neutral fat can be metabolized, it must be hydrolyzed to its component parts. hydrolysis 【19-->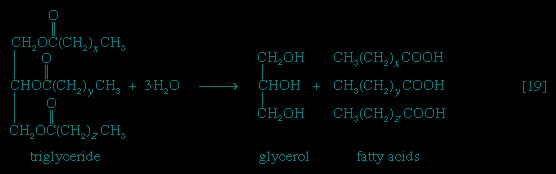 】 is effected by intracellular enzymes or gut enzymes, and forms phase I of fat catabolism. Letters x, y, and z represent the number of −CH2− groups in the fatty acid molecules.
】 is effected by intracellular enzymes or gut enzymes, and forms phase I of fat catabolism. Letters x, y, and z represent the number of −CH2− groups in the fatty acid molecules.
 】 is effected by intracellular enzymes or gut enzymes, and forms phase I of fat catabolism. Letters x, y, and z represent the number of −CH2− groups in the fatty acid molecules.
】 is effected by intracellular enzymes or gut enzymes, and forms phase I of fat catabolism. Letters x, y, and z represent the number of −CH2− groups in the fatty acid molecules.
As is apparent from 【19--> 】, the three molecules of fatty acid released from the triglyceride need not be identical; a fatty acid usually contains 16 or 18 carbon atoms but may also be unsaturated; that is, containing one or more double bonds (−CH=CH−). Only the fate of saturated fatty acids, of the type CH3(CH2)nCOOH (n most commonly is an even number), is dealt with here.
】, the three molecules of fatty acid released from the triglyceride need not be identical; a fatty acid usually contains 16 or 18 carbon atoms but may also be unsaturated; that is, containing one or more double bonds (−CH=CH−). Only the fate of saturated fatty acids, of the type CH3(CH2)nCOOH (n most commonly is an even number), is dealt with here.
 】, the three molecules of fatty acid released from the triglyceride need not be identical; a fatty acid usually contains 16 or 18 carbon atoms but may also be unsaturated; that is, containing one or more double bonds (−CH=CH−). Only the fate of saturated fatty acids, of the type CH3(CH2)nCOOH (n most commonly is an even number), is dealt with here.
】, the three molecules of fatty acid released from the triglyceride need not be identical; a fatty acid usually contains 16 or 18 carbon atoms but may also be unsaturated; that is, containing one or more double bonds (−CH=CH−). Only the fate of saturated fatty acids, of the type CH3(CH2)nCOOH (n most commonly is an even number), is dealt with here.Fate of glycerol
 Special Comp-->
Special Comp-->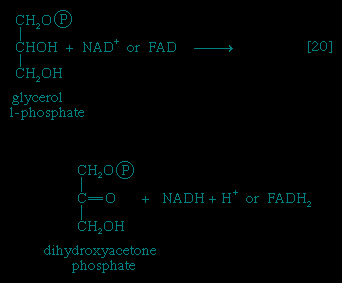 It requires but two reactions to channel glycerol into a catabolic pathway (see Figure 2-->
It requires but two reactions to channel glycerol into a catabolic pathway (see Figure 2--> ). In a reaction catalyzed by glycerolkinase, ATP is used to phosphorylate glycerol; the products are glycerol 1-phosphate and ADP. Glycerol 1-phosphate is then oxidized to dihydroxyacetone phosphate 【20-->
). In a reaction catalyzed by glycerolkinase, ATP is used to phosphorylate glycerol; the products are glycerol 1-phosphate and ADP. Glycerol 1-phosphate is then oxidized to dihydroxyacetone phosphate 【20--> 】, an intermediate of glycolysis. The reaction is catalyzed by either a soluble (cytoplasmic) enzyme, glycerolphosphate dehydrogenase, or a similar enzyme present in the mitochondria. In addition to their different locations, the two dehydrogenase enzymes differ in that a different coenzyme accepts the electrons removed from glycerol 1-phosphate. In the case of the cytoplasmic enzyme, NAD+ accepts the electrons (and is reduced to NADH + H+); in the case of the mitochondrial enzyme, flavin adenine dinucleotide (FAD) accepts the electrons (and is reduced to FADH2).
】, an intermediate of glycolysis. The reaction is catalyzed by either a soluble (cytoplasmic) enzyme, glycerolphosphate dehydrogenase, or a similar enzyme present in the mitochondria. In addition to their different locations, the two dehydrogenase enzymes differ in that a different coenzyme accepts the electrons removed from glycerol 1-phosphate. In the case of the cytoplasmic enzyme, NAD+ accepts the electrons (and is reduced to NADH + H+); in the case of the mitochondrial enzyme, flavin adenine dinucleotide (FAD) accepts the electrons (and is reduced to FADH2).
Fate of fatty acids
Formation of fatty acyl coenzyme A molecules
As with sugars, the release of energy from fatty acids necessitates an initial investment of ATP. A problem unique to fats is a consequence of the low solubility in water of most fatty acids. Their catabolism requires mechanisms that fragment them in a controlled and stepwise manner. The mechanism involves a coenzyme for the transfer of an acyl group (e.g., CH3C∣=O), namely, coenzyme A. The functional portion of this complex molecule is the sulfhydryl (−SH) group at one end. The coenzyme is often identified as CoA−SH (see step 【21-->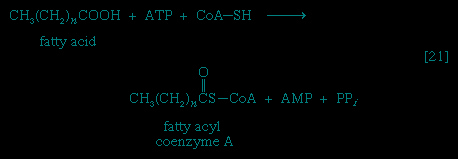 】). The organized and stepwise degradation of fatty acids linked to coenzyme A is ensured because the necessary enzymes are sequestered in particulate structures. In microorganisms these enzymes are associated with cell membranes, and in higher organisms with mitochondria.
】). The organized and stepwise degradation of fatty acids linked to coenzyme A is ensured because the necessary enzymes are sequestered in particulate structures. In microorganisms these enzymes are associated with cell membranes, and in higher organisms with mitochondria.
 】). The organized and stepwise degradation of fatty acids linked to coenzyme A is ensured because the necessary enzymes are sequestered in particulate structures. In microorganisms these enzymes are associated with cell membranes, and in higher organisms with mitochondria.
】). The organized and stepwise degradation of fatty acids linked to coenzyme A is ensured because the necessary enzymes are sequestered in particulate structures. In microorganisms these enzymes are associated with cell membranes, and in higher organisms with mitochondria.
Fatty acids are linked to coenzyme A (CoA−SH) in one of two main ways. In higher organisms, enzymes in the cytoplasm called thiokinases catalyze the linkage of fatty acids with CoA−SH to form a compound that can be called a fatty acyl coenzyme A 【21--> 】. This step requires ATP, which is split to AMP and inorganic pyrophosphate (PPi) in the process.
】. This step requires ATP, which is split to AMP and inorganic pyrophosphate (PPi) in the process.
 】. This step requires ATP, which is split to AMP and inorganic pyrophosphate (PPi) in the process.
】. This step requires ATP, which is split to AMP and inorganic pyrophosphate (PPi) in the process.In this series of reactions, n indicates the number of hydrocarbon units (−CH2−) in the molecule. Because most tissues contain highly active pyrophosphatase enzymes 【21a-->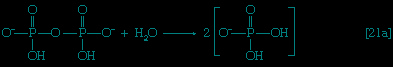 】, which catalyze the virtually irreversible hydrolysis of inorganic pyrophosphate (PPi) to two molecules of inorganic phosphate (Pi), reaction 【21-->
】, which catalyze the virtually irreversible hydrolysis of inorganic pyrophosphate (PPi) to two molecules of inorganic phosphate (Pi), reaction 【21--> 】 proceeds overwhelmingly to completion; i.e., from left to right.
】 proceeds overwhelmingly to completion; i.e., from left to right.
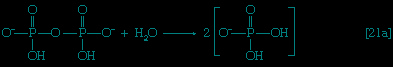 】, which catalyze the virtually irreversible hydrolysis of inorganic pyrophosphate (PPi) to two molecules of inorganic phosphate (Pi), reaction 【21-->
】, which catalyze the virtually irreversible hydrolysis of inorganic pyrophosphate (PPi) to two molecules of inorganic phosphate (Pi), reaction 【21--> 】 proceeds overwhelmingly to completion; i.e., from left to right.
】 proceeds overwhelmingly to completion; i.e., from left to right.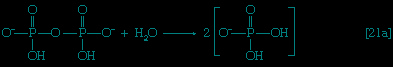
Although fatty acids are activated in this way, the acyl coenzyme A derivatives that are formed must be transported to the enzyme complex that effects their oxidation. Activation occurs in the cytoplasm, but, in animal cells, oxidation takes place in the mitochondria. The transfer of fatty acyl coenzyme A across the mitochondrial membrane is effected by the enzyme carnitine, a nitrogen-containing small hydroxy acid of the formula (CH3)3NCH2CH(OH)CH2COO-. The −OH group within the carnitine molecule accepts the acyl group of fatty acyl coenzyme A,
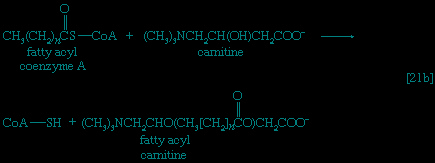
forming acyl carnitine, which can cross the inner membrane of the mitochondrion and there return the acyl group to coenzyme A.
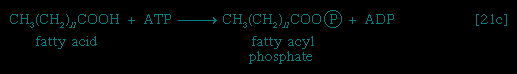
Special Comp-->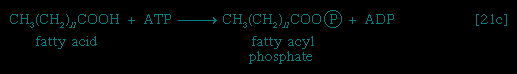 Special Comp-->
Special Comp-->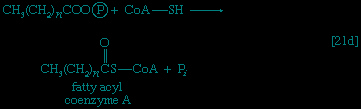
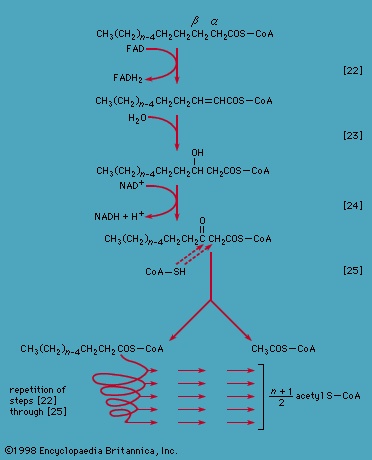 These reactions are catalyzed by the enzyme carnitine acyl transferase. Defects in this enzyme or in the carnitine carrier are inborn errors of metabolism. In obligate anaerobic bacteria the linkage of fatty acids to coenzyme A may require the formation of a fatty acyl phosphate, i.e., the phosphorylation of the fatty acid using ATP; ADP is also a product 【21c-->
These reactions are catalyzed by the enzyme carnitine acyl transferase. Defects in this enzyme or in the carnitine carrier are inborn errors of metabolism. In obligate anaerobic bacteria the linkage of fatty acids to coenzyme A may require the formation of a fatty acyl phosphate, i.e., the phosphorylation of the fatty acid using ATP; ADP is also a product 【21c-->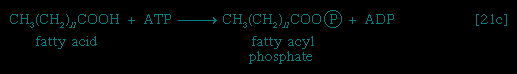 】. The fatty acyl moiety 【CH3(CH2)nCOO-】 is then transferred to coenzyme A 【21d-->
】. The fatty acyl moiety 【CH3(CH2)nCOO-】 is then transferred to coenzyme A 【21d-->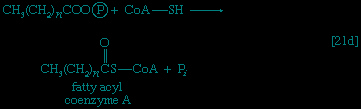 】, forming a fatty acyl coenzyme A compound and Pi. In either case, it is the fatty acyl coenzyme A molecules that are fragmented in the sequence of events summarized in Figure 5-->
】, forming a fatty acyl coenzyme A compound and Pi. In either case, it is the fatty acyl coenzyme A molecules that are fragmented in the sequence of events summarized in Figure 5--> .
.
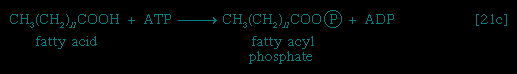 Special Comp-->
Special Comp-->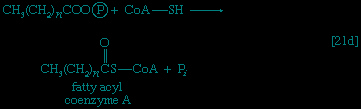
 These reactions are catalyzed by the enzyme carnitine acyl transferase. Defects in this enzyme or in the carnitine carrier are inborn errors of metabolism. In obligate anaerobic bacteria the linkage of fatty acids to coenzyme A may require the formation of a fatty acyl phosphate, i.e., the phosphorylation of the fatty acid using ATP; ADP is also a product 【21c-->
These reactions are catalyzed by the enzyme carnitine acyl transferase. Defects in this enzyme or in the carnitine carrier are inborn errors of metabolism. In obligate anaerobic bacteria the linkage of fatty acids to coenzyme A may require the formation of a fatty acyl phosphate, i.e., the phosphorylation of the fatty acid using ATP; ADP is also a product 【21c-->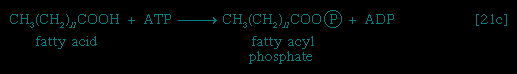 】. The fatty acyl moiety 【CH3(CH2)nCOO-】 is then transferred to coenzyme A 【21d-->
】. The fatty acyl moiety 【CH3(CH2)nCOO-】 is then transferred to coenzyme A 【21d-->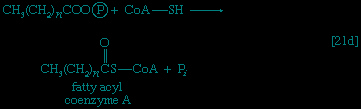 】, forming a fatty acyl coenzyme A compound and Pi. In either case, it is the fatty acyl coenzyme A molecules that are fragmented in the sequence of events summarized in Figure 5-->
】, forming a fatty acyl coenzyme A compound and Pi. In either case, it is the fatty acyl coenzyme A molecules that are fragmented in the sequence of events summarized in Figure 5--> .
.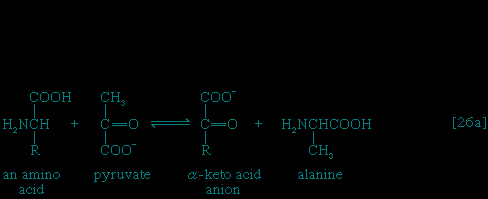
Fragmentation of fatty acyl coenzyme A molecules
Special Comp-->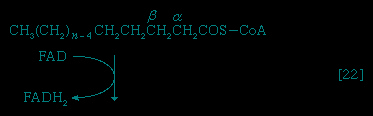
 Initially (step 【22】-->
Initially (step 【22】-->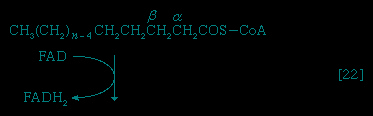 , Figure 5-->
, Figure 5--> ) two hydrogen atoms are lost from the fatty acyl coenzyme A, resulting in the formation of an unsaturated fatty acyl coenzyme A (i.e., with a double bond, −CH=CH−) between the α- and β-carbons of the acyl moiety.
) two hydrogen atoms are lost from the fatty acyl coenzyme A, resulting in the formation of an unsaturated fatty acyl coenzyme A (i.e., with a double bond, −CH=CH−) between the α- and β-carbons of the acyl moiety.
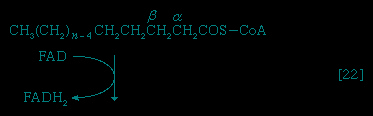
 Initially (step 【22】-->
Initially (step 【22】-->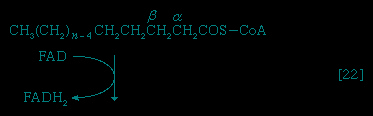 , Figure 5-->
, Figure 5--> ) two hydrogen atoms are lost from the fatty acyl coenzyme A, resulting in the formation of an unsaturated fatty acyl coenzyme A (i.e., with a double bond, −CH=CH−) between the α- and β-carbons of the acyl moiety.
) two hydrogen atoms are lost from the fatty acyl coenzyme A, resulting in the formation of an unsaturated fatty acyl coenzyme A (i.e., with a double bond, −CH=CH−) between the α- and β-carbons of the acyl moiety.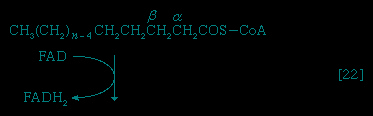
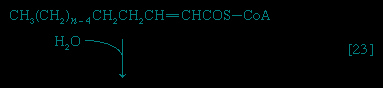
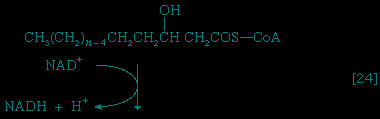

(The α-carbon is the one closest to the carboxyl 【−COOH】 group of a fatty acid; the next closest is the β-, and so on to the end of the hydrocarbon chain.) The hydrogen atoms are accepted by the coenzyme FAD (flavin adenine dinucleotide), which is reduced to FADH2. The product of step 【22】, α,β-unsaturated fatty acyl coenzyme A, is enzymatically hydrated 【23-->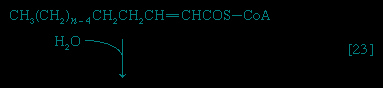 】; i.e., water is added across the double bond. The product, called a β-hydroxyacyl coenzyme A, can again be oxidized in an enzyme-catalyzed reaction 【24】; the electrons removed are accepted by NAD+. The product is called a β-ketoacyl coenzyme A.
】; i.e., water is added across the double bond. The product, called a β-hydroxyacyl coenzyme A, can again be oxidized in an enzyme-catalyzed reaction 【24】; the electrons removed are accepted by NAD+. The product is called a β-ketoacyl coenzyme A.
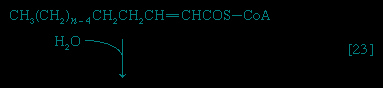 】; i.e., water is added across the double bond. The product, called a β-hydroxyacyl coenzyme A, can again be oxidized in an enzyme-catalyzed reaction 【24】; the electrons removed are accepted by NAD+. The product is called a β-ketoacyl coenzyme A.
】; i.e., water is added across the double bond. The product, called a β-hydroxyacyl coenzyme A, can again be oxidized in an enzyme-catalyzed reaction 【24】; the electrons removed are accepted by NAD+. The product is called a β-ketoacyl coenzyme A.The next enzymatic step 【25--> 】 enables the energy invested in step 【21-->
】 enables the energy invested in step 【21--> 】 to be conserved. The β-ketoacyl coenzyme A that is the product of reaction 【24-->
】 to be conserved. The β-ketoacyl coenzyme A that is the product of reaction 【24-->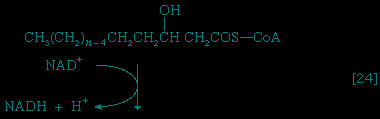 】 is split, not by water but by coenzyme A. The process, called thiolysis (as distinct from hydrolysis), yields the two-carbon fragment acetyl coenzyme A and a fatty acyl coenzyme A having two fewer carbon atoms than the molecule that underwent reaction 【22-->
】 is split, not by water but by coenzyme A. The process, called thiolysis (as distinct from hydrolysis), yields the two-carbon fragment acetyl coenzyme A and a fatty acyl coenzyme A having two fewer carbon atoms than the molecule that underwent reaction 【22-->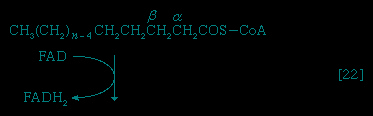 】; otherwise the two are similar.
】; otherwise the two are similar.
 】 enables the energy invested in step 【21-->
】 enables the energy invested in step 【21--> 】 to be conserved. The β-ketoacyl coenzyme A that is the product of reaction 【24-->
】 to be conserved. The β-ketoacyl coenzyme A that is the product of reaction 【24-->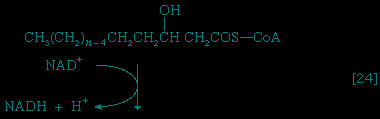 】 is split, not by water but by coenzyme A. The process, called thiolysis (as distinct from hydrolysis), yields the two-carbon fragment acetyl coenzyme A and a fatty acyl coenzyme A having two fewer carbon atoms than the molecule that underwent reaction 【22-->
】 is split, not by water but by coenzyme A. The process, called thiolysis (as distinct from hydrolysis), yields the two-carbon fragment acetyl coenzyme A and a fatty acyl coenzyme A having two fewer carbon atoms than the molecule that underwent reaction 【22-->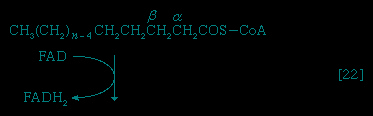 】; otherwise the two are similar.
】; otherwise the two are similar.Special Comp-->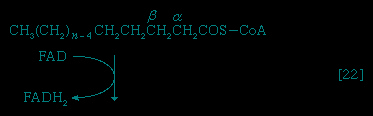
 The shortened fatty acyl coenzyme A molecule now undergoes the sequence of reactions again, beginning with the dehydrogenation step 【22-->
The shortened fatty acyl coenzyme A molecule now undergoes the sequence of reactions again, beginning with the dehydrogenation step 【22-->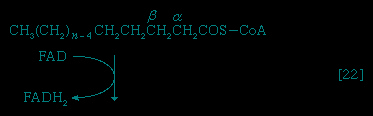 】, and another two-carbon fragment is removed as acetyl coenzyme A. With each passage through the process of fatty acid oxidation, the fatty acid loses a two-carbon fragment as acetyl coenzyme A and two pairs of hydrogen atoms to specific acceptors. The 16-carbon fatty acid, palmitic acid, for example, undergoes a total of seven such cycles, yielding eight molecules of acetyl coenzyme A and 14 pairs of hydrogen atoms, seven of which appear in the form of FADH2 and seven in the form of NADH + H+. The reduced coenzymes, FADH2 and reduced NAD+, are reoxidized when the electrons pass through the electron transport chain, with concomitant formation of ATP (see below Biological energy transduction (metabolism)). In anaerobes, organic molecules and not oxygen are electron acceptors; thus the yield of ATP is reduced. In all organisms, however, the acetyl coenzyme A formed from the breakdown of fatty acids joins that arising from the catabolism of carbohydrates (see below The oxidation of pyruvate (metabolism)) and many amino acids (see below The catabolism of proteins: Oxidation of the carbon skeleton (metabolism)); Figure 2-->
】, and another two-carbon fragment is removed as acetyl coenzyme A. With each passage through the process of fatty acid oxidation, the fatty acid loses a two-carbon fragment as acetyl coenzyme A and two pairs of hydrogen atoms to specific acceptors. The 16-carbon fatty acid, palmitic acid, for example, undergoes a total of seven such cycles, yielding eight molecules of acetyl coenzyme A and 14 pairs of hydrogen atoms, seven of which appear in the form of FADH2 and seven in the form of NADH + H+. The reduced coenzymes, FADH2 and reduced NAD+, are reoxidized when the electrons pass through the electron transport chain, with concomitant formation of ATP (see below Biological energy transduction (metabolism)). In anaerobes, organic molecules and not oxygen are electron acceptors; thus the yield of ATP is reduced. In all organisms, however, the acetyl coenzyme A formed from the breakdown of fatty acids joins that arising from the catabolism of carbohydrates (see below The oxidation of pyruvate (metabolism)) and many amino acids (see below The catabolism of proteins: Oxidation of the carbon skeleton (metabolism)); Figure 2--> shows the interrelationships.
shows the interrelationships.
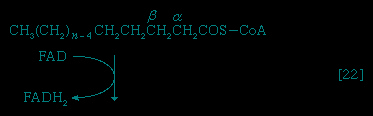
 The shortened fatty acyl coenzyme A molecule now undergoes the sequence of reactions again, beginning with the dehydrogenation step 【22-->
The shortened fatty acyl coenzyme A molecule now undergoes the sequence of reactions again, beginning with the dehydrogenation step 【22-->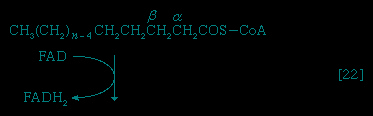 】, and another two-carbon fragment is removed as acetyl coenzyme A. With each passage through the process of fatty acid oxidation, the fatty acid loses a two-carbon fragment as acetyl coenzyme A and two pairs of hydrogen atoms to specific acceptors. The 16-carbon fatty acid, palmitic acid, for example, undergoes a total of seven such cycles, yielding eight molecules of acetyl coenzyme A and 14 pairs of hydrogen atoms, seven of which appear in the form of FADH2 and seven in the form of NADH + H+. The reduced coenzymes, FADH2 and reduced NAD+, are reoxidized when the electrons pass through the electron transport chain, with concomitant formation of ATP (see below Biological energy transduction (metabolism)). In anaerobes, organic molecules and not oxygen are electron acceptors; thus the yield of ATP is reduced. In all organisms, however, the acetyl coenzyme A formed from the breakdown of fatty acids joins that arising from the catabolism of carbohydrates (see below The oxidation of pyruvate (metabolism)) and many amino acids (see below The catabolism of proteins: Oxidation of the carbon skeleton (metabolism)); Figure 2-->
】, and another two-carbon fragment is removed as acetyl coenzyme A. With each passage through the process of fatty acid oxidation, the fatty acid loses a two-carbon fragment as acetyl coenzyme A and two pairs of hydrogen atoms to specific acceptors. The 16-carbon fatty acid, palmitic acid, for example, undergoes a total of seven such cycles, yielding eight molecules of acetyl coenzyme A and 14 pairs of hydrogen atoms, seven of which appear in the form of FADH2 and seven in the form of NADH + H+. The reduced coenzymes, FADH2 and reduced NAD+, are reoxidized when the electrons pass through the electron transport chain, with concomitant formation of ATP (see below Biological energy transduction (metabolism)). In anaerobes, organic molecules and not oxygen are electron acceptors; thus the yield of ATP is reduced. In all organisms, however, the acetyl coenzyme A formed from the breakdown of fatty acids joins that arising from the catabolism of carbohydrates (see below The oxidation of pyruvate (metabolism)) and many amino acids (see below The catabolism of proteins: Oxidation of the carbon skeleton (metabolism)); Figure 2--> shows the interrelationships.
shows the interrelationships.Fatty acids with an odd number of carbon atoms are relatively rare in nature but may arise during microbial fermentations or through the oxidation of amino acids such as valine and isoleucine. They may be fragmented through repeated cycles of steps 【22】 to 【25】 until the final five-carbon acyl coenzyme A is split into acetyl coenzyme A and propionyl coenzyme A, which has three carbon atoms. In many bacteria this propionyl coenzyme A can be transformed either to acetyl coenzyme A and carbon dioxide or to pyruvate. In other microorganisms and in animals propionyl coenzyme A has a different fate: carbon dioxide is added to propionyl coenzyme A in a reaction requiring ATP. The product, methylmalonyl coenzyme A, has four carbon atoms; the molecule undergoes a rearrangement, forming succinyl coenzyme A, which is an intermediate of the TCA cycle.
The catabolism of proteins (protein)
The amino acids (amino acid) derived from proteins function primarily as the precursors, or building blocks, for the cell's own proteins and (unlike lipids and carbohydrates) are not primarily a source of energy. Many microorganisms, on the other hand, can grow by using amino acids as the sole carbon and nitrogen source. Under these conditions these microorganisms derive from the amino acids all of their required energy and all of the precursors of the macromolecules that comprise the components of their cells. Moreover, it has been calculated that a man of average weight (70 kilograms, or 154 pounds) turns over about 0.4 kilogram of protein per day. About 0.1 kilogram is degraded and replaced by dietary amino acids; the remaining 0.3 kilogram is recycled as part of the dynamic state of cell constituents. The cells of plants contain and metabolize many amino acids in addition to the 20 or so that are normally found in proteins. A complete discussion of these special pathways is outside the scope of this article, however.
Before proteins can enter cells, the bonds linking adjacent amino acids (peptide bonds) must be hydrolyzed; this process releases the amino acids constituting the protein. The utilization of dietary proteins thus requires the operation of extracellular digestive enzymes; i.e., enzymes outside the cell. Many microorganisms secrete such enzymes into the nutrient media in which they are growing; animals secrete them into the gut. The turnover of proteins within cells, on the other hand, requires the functioning of intracellular enzymes that catalyze the splitting of the peptide bonds linking adjacent amino acids; little is known about the mechanism involved.
Amino acids may be described by the general formula RCH(NH2)COOH, or RCH(NH3+)COO-, in which R represents a specific chemical moiety. The catabolic fate of amino acids involves (1) removal of nitrogen, (2) disposal of nitrogen, and (3) oxidation of the remaining carbon skeleton.
Removal of nitrogen
The removal of the amino group (-NH2) generally constitutes the first stage in amino-acid catabolism. The amino group usually is initially transferred to the anion of one of three different α-keto acids (i.e., of the general structure RCOCOO-): pyruvate, which is an intermediate of carbohydrate fragmentation; or oxaloacetate or α-oxoglutarate, both intermediates of the TCA cycle. The products are alanine, aspartate, and glutamate (reactions 【26a--> , b-->
, b--> , and c-->
, and c-->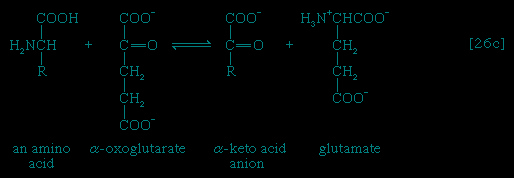 】).
】).
 , b-->
, b--> , and c-->
, and c--> 】).
】).


Since the effect of these reactions is to produce n amino acids and n keto acids from n different amino acids and n different keto acids, no net reduction in the nitrogen content of the system has yet been achieved. The elimination of nitrogen occurs in a variety of ways.
In many microorganisms, ammonia (NH3) can be removed from aspartate via a reaction catalyzed by aspartase 【27-->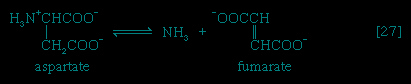 】; the other product, fumarate, is an intermediate of the TCA cycle.
】; the other product, fumarate, is an intermediate of the TCA cycle.
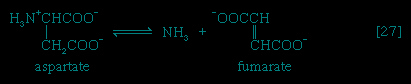 】; the other product, fumarate, is an intermediate of the TCA cycle.
】; the other product, fumarate, is an intermediate of the TCA cycle.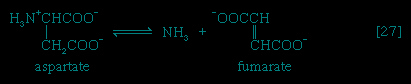
A quantitatively more important route is that catalyzed by glutamate dehydrogenase, in which the glutamate formed in 【26c--> 】 is oxidized to α-oxoglutarate, another TCA cycle intermediate 【28-->
】 is oxidized to α-oxoglutarate, another TCA cycle intermediate 【28-->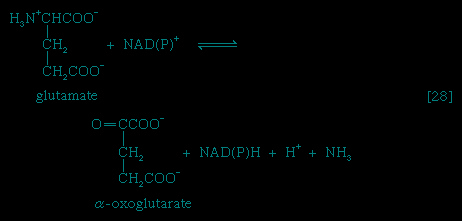 】. Either NADP+ or both NADP+ and NAD+ may serve as the hydrogen or electron acceptor, depending on the organism; and some organisms synthesize two enzymes, one of which prefers NADP+ and the other NAD+. In reaction 【28-->
】. Either NADP+ or both NADP+ and NAD+ may serve as the hydrogen or electron acceptor, depending on the organism; and some organisms synthesize two enzymes, one of which prefers NADP+ and the other NAD+. In reaction 【28--> 】, NAD(P)+ is used to indicate that either NAD+, NADP+, or both may serve as the electron acceptor.
】, NAD(P)+ is used to indicate that either NAD+, NADP+, or both may serve as the electron acceptor.
 】 is oxidized to α-oxoglutarate, another TCA cycle intermediate 【28-->
】 is oxidized to α-oxoglutarate, another TCA cycle intermediate 【28--> 】. Either NADP+ or both NADP+ and NAD+ may serve as the hydrogen or electron acceptor, depending on the organism; and some organisms synthesize two enzymes, one of which prefers NADP+ and the other NAD+. In reaction 【28-->
】. Either NADP+ or both NADP+ and NAD+ may serve as the hydrogen or electron acceptor, depending on the organism; and some organisms synthesize two enzymes, one of which prefers NADP+ and the other NAD+. In reaction 【28--> 】, NAD(P)+ is used to indicate that either NAD+, NADP+, or both may serve as the electron acceptor.
】, NAD(P)+ is used to indicate that either NAD+, NADP+, or both may serve as the electron acceptor.The occurrence of the transfer reactions 【26-->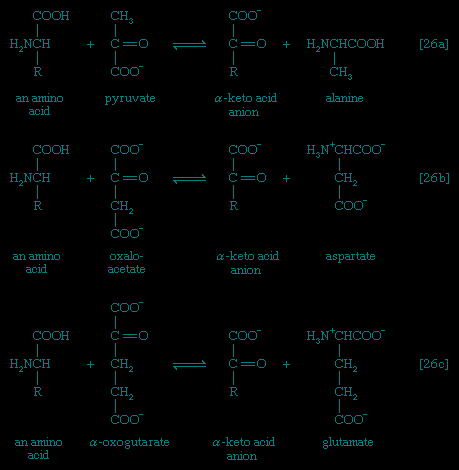 】 and either step 【27-->
】 and either step 【27-->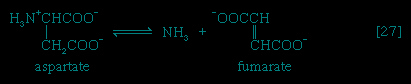 】 or, more importantly, step 【28-->
】 or, more importantly, step 【28--> 】 allows the channeling of many amino acids into a common pathway by which nitrogen can be eliminated as ammonia.
】 allows the channeling of many amino acids into a common pathway by which nitrogen can be eliminated as ammonia.
 】 and either step 【27-->
】 and either step 【27-->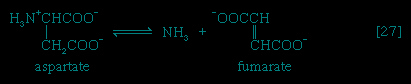 】 or, more importantly, step 【28-->
】 or, more importantly, step 【28--> 】 allows the channeling of many amino acids into a common pathway by which nitrogen can be eliminated as ammonia.
】 allows the channeling of many amino acids into a common pathway by which nitrogen can be eliminated as ammonia.
Disposal of nitrogen
In animals that excrete ammonia as the main nitrogenous waste product (e.g., some marine invertebrates, crustaceans), it is derived from nitrogen transfer reactions 【26--> 】 and oxidation via glutamate dehydrogenase 【28-->
】 and oxidation via glutamate dehydrogenase 【28--> 】 as described above for microorganisms. Because ammonia is toxic to cells, however, it is detoxified as it forms. This process involves an enzyme-catalyzed reaction between ammonia and a molecule of glutamate; ATP provides the energy for the reaction, which results in the formation of glutamine, ADP, and inorganic phosphate 【29-->
】 as described above for microorganisms. Because ammonia is toxic to cells, however, it is detoxified as it forms. This process involves an enzyme-catalyzed reaction between ammonia and a molecule of glutamate; ATP provides the energy for the reaction, which results in the formation of glutamine, ADP, and inorganic phosphate 【29-->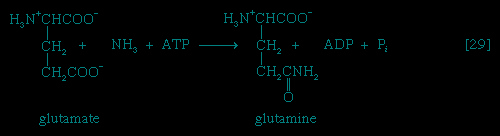 】. This reaction 【29-->
】. This reaction 【29--> 】 is catalyzed by glutamine synthetase, which is subject to a variety of metabolic controls. The glutamine thus formed gives up the amide nitrogen in the kidney tubules. As a result glutamate is formed once again, and ammonia is released into the urine.
】 is catalyzed by glutamine synthetase, which is subject to a variety of metabolic controls. The glutamine thus formed gives up the amide nitrogen in the kidney tubules. As a result glutamate is formed once again, and ammonia is released into the urine.
 】 and oxidation via glutamate dehydrogenase 【28-->
】 and oxidation via glutamate dehydrogenase 【28--> 】 as described above for microorganisms. Because ammonia is toxic to cells, however, it is detoxified as it forms. This process involves an enzyme-catalyzed reaction between ammonia and a molecule of glutamate; ATP provides the energy for the reaction, which results in the formation of glutamine, ADP, and inorganic phosphate 【29-->
】 as described above for microorganisms. Because ammonia is toxic to cells, however, it is detoxified as it forms. This process involves an enzyme-catalyzed reaction between ammonia and a molecule of glutamate; ATP provides the energy for the reaction, which results in the formation of glutamine, ADP, and inorganic phosphate 【29--> 】. This reaction 【29-->
】. This reaction 【29--> 】 is catalyzed by glutamine synthetase, which is subject to a variety of metabolic controls. The glutamine thus formed gives up the amide nitrogen in the kidney tubules. As a result glutamate is formed once again, and ammonia is released into the urine.
】 is catalyzed by glutamine synthetase, which is subject to a variety of metabolic controls. The glutamine thus formed gives up the amide nitrogen in the kidney tubules. As a result glutamate is formed once again, and ammonia is released into the urine.
 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> In terrestrial reptiles and birds, uric acid rather than glutamate is the compound with which nitrogen combines to form a nontoxic substance for transfer to the kidney tubules. Uric acid is formed by a complex pathway that begins with ribose 5-phosphate and during which a so-called purine skeleton (see Figure 11-->
In terrestrial reptiles and birds, uric acid rather than glutamate is the compound with which nitrogen combines to form a nontoxic substance for transfer to the kidney tubules. Uric acid is formed by a complex pathway that begins with ribose 5-phosphate and during which a so-called purine skeleton (see Figure 11--> ) is formed; in the course of this process, nitrogen atoms from glutamine and the amino acids aspartic acid and glycine are incorporated into the skeleton. These nitrogen donors are derived from other amino acids via amino group transfer 【26-->
) is formed; in the course of this process, nitrogen atoms from glutamine and the amino acids aspartic acid and glycine are incorporated into the skeleton. These nitrogen donors are derived from other amino acids via amino group transfer 【26--> 】 and the reaction catalyzed by glutamine synthetase 【29-->
】 and the reaction catalyzed by glutamine synthetase 【29--> 】.
】.In most fishes, amphibians, and mammals, nitrogen is detoxified in the liver and excreted as urea, a readily soluble and harmless product. The sequence leading to the formation of urea, commonly called the urea cycle, is summarized as follows: Ammonia, formed from glutamate and NAD+ in the liver mitochondria (reaction 【28--> 】), reacts with carbon dioxide and ATP to form carbamoyl phosphate, ADP, and inorganic phosphate, as shown in reaction 【30-->
】), reacts with carbon dioxide and ATP to form carbamoyl phosphate, ADP, and inorganic phosphate, as shown in reaction 【30-->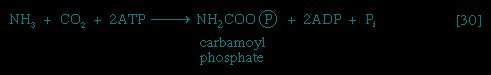 】.
】.
 】), reacts with carbon dioxide and ATP to form carbamoyl phosphate, ADP, and inorganic phosphate, as shown in reaction 【30-->
】), reacts with carbon dioxide and ATP to form carbamoyl phosphate, ADP, and inorganic phosphate, as shown in reaction 【30-->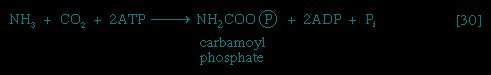 】.
】.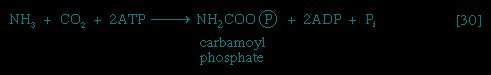
The reaction is catalyzed by carbamoyl phosphate synthetase. The carbamoyl moiety of carbamoyl phosphate (NH2CO−) is transferred to ornithine, an amino acid, in a reaction catalyzed by ornithine transcarbamoylase; the products are citrulline and inorganic phosphate 【31-->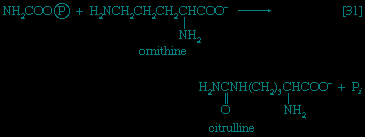 】. Citrulline and aspartate formed from amino acids via step 【26b-->
】. Citrulline and aspartate formed from amino acids via step 【26b--> 】 react to form argininosuccinate 【32-->
】 react to form argininosuccinate 【32-->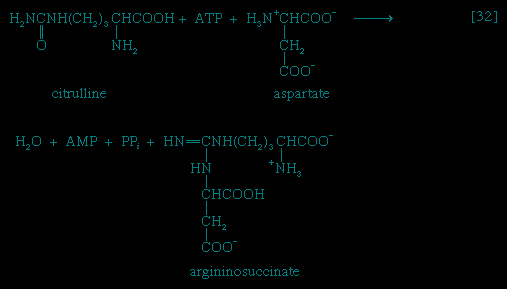 】; argininosuccinic acid synthetase catalyzes the reaction. Argininosuccinate splits into fumarate and arginine during a reaction
】; argininosuccinic acid synthetase catalyzes the reaction. Argininosuccinate splits into fumarate and arginine during a reaction
 】. Citrulline and aspartate formed from amino acids via step 【26b-->
】. Citrulline and aspartate formed from amino acids via step 【26b--> 】 react to form argininosuccinate 【32-->
】 react to form argininosuccinate 【32--> 】; argininosuccinic acid synthetase catalyzes the reaction. Argininosuccinate splits into fumarate and arginine during a reaction
】; argininosuccinic acid synthetase catalyzes the reaction. Argininosuccinate splits into fumarate and arginine during a reaction

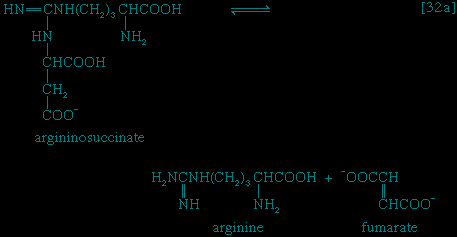
catalyzed by argininosuccinase 【32a--> 】. In the final step of the urea cycle, arginine, in a reaction catalyzed by arginase, is hydrolyzed 【33-->
】. In the final step of the urea cycle, arginine, in a reaction catalyzed by arginase, is hydrolyzed 【33-->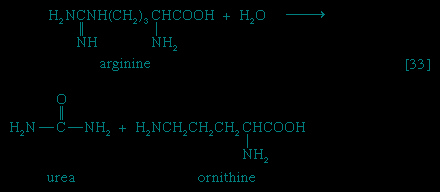 】. Urea and ornithine are the products; ornithine thus is available to initiate another cycle beginning at step 【31-->
】. Urea and ornithine are the products; ornithine thus is available to initiate another cycle beginning at step 【31--> 】.
】.
 】. In the final step of the urea cycle, arginine, in a reaction catalyzed by arginase, is hydrolyzed 【33-->
】. In the final step of the urea cycle, arginine, in a reaction catalyzed by arginase, is hydrolyzed 【33--> 】. Urea and ornithine are the products; ornithine thus is available to initiate another cycle beginning at step 【31-->
】. Urea and ornithine are the products; ornithine thus is available to initiate another cycle beginning at step 【31--> 】.
】.
Oxidation of the carbon skeleton
 As indicated in Figure 2-->
As indicated in Figure 2--> , the carbon skeletons of amino acids (i.e., the portion of the molecule remaining after the removal of nitrogen) are fragmented to form only a few end products; all of them are intermediates of either glycolysis or the TCA cycle. The number and complexity of the catabolic steps by which each amino acid arrives at its catabolic end point reflects the chemical complexity of that amino acid. Thus, in the case of alanine, only the amino group must be removed to yield pyruvate; the amino acid threonine, on the other hand, must be transformed successively to the amino acids glycine and serine before pyruvate is formed. The fragmentation of leucine to acetyl coenzyme A involves seven steps; that of tryptophan to the same end product requires 11. (A detailed discussion of the events that enable each of the 20 commonly occurring amino acids to enter central metabolic pathways is beyond the scope of this article.)
, the carbon skeletons of amino acids (i.e., the portion of the molecule remaining after the removal of nitrogen) are fragmented to form only a few end products; all of them are intermediates of either glycolysis or the TCA cycle. The number and complexity of the catabolic steps by which each amino acid arrives at its catabolic end point reflects the chemical complexity of that amino acid. Thus, in the case of alanine, only the amino group must be removed to yield pyruvate; the amino acid threonine, on the other hand, must be transformed successively to the amino acids glycine and serine before pyruvate is formed. The fragmentation of leucine to acetyl coenzyme A involves seven steps; that of tryptophan to the same end product requires 11. (A detailed discussion of the events that enable each of the 20 commonly occurring amino acids to enter central metabolic pathways is beyond the scope of this article.)The combustion of food materials
Although the pathways for fragmentation of food materials effect the conversion of a large variety of relatively complex starting materials into only a few simpler intermediates of central metabolic routes—mainly pyruvate, acetyl coenzyme A, and a few intermediates of the TCA cycle—their operation releases but a fraction of the energy contained in the materials. The reason is that, in the fermentation process, catabolic intermediates serve also as the terminal acceptors of the reducing equivalents (hydrogen atoms or electrons) that are removed during the oxidation of food; the end products thus may be at the same oxidation level and may contain equivalent numbers of carbon, hydrogen, and oxygen atoms, as the material that was catabolized by a fermentative route. The necessity for pyruvate, for example, to act as hydrogen acceptor in the fermentation of glucose to lactate (see reactions 【1--> , 2-->
, 2--> , 3-->
, 3--> , 4-->
, 4--> , 5-->
, 5--> , 6-->
, 6--> , 7-->
, 7--> , 8-->
, 8-->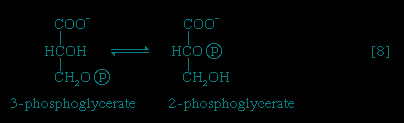 , 9-->
, 9-->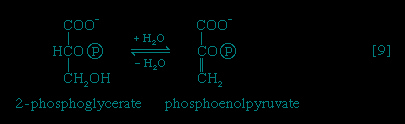 , and 10-->
, and 10--> , 11-->
, 11--> 】) results in the conservation of all the component atoms of the glucose molecule in the form of lactate. The consequent release of energy as ATP (in steps 【7-->
】) results in the conservation of all the component atoms of the glucose molecule in the form of lactate. The consequent release of energy as ATP (in steps 【7--> 】 and 【10-->
】 and 【10--> 】) is thus small.
】) is thus small.
 , 2-->
, 2--> , 3-->
, 3--> , 4-->
, 4--> , 5-->
, 5--> , 6-->
, 6--> , 7-->
, 7--> , 8-->
, 8-->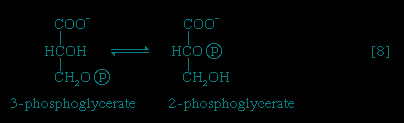 , 9-->
, 9-->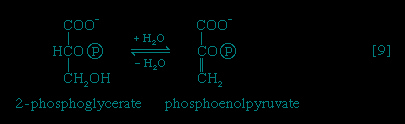 , and 10-->
, and 10--> , 11-->
, 11--> 】) results in the conservation of all the component atoms of the glucose molecule in the form of lactate. The consequent release of energy as ATP (in steps 【7-->
】) results in the conservation of all the component atoms of the glucose molecule in the form of lactate. The consequent release of energy as ATP (in steps 【7--> 】 and 【10-->
】 and 【10--> 】) is thus small.
】) is thus small.A more favourable situation arises if the reducing equivalents formed by oxidation of nutrients (nutrient) can be passed on to an inorganic acceptor such as oxygen. In this case, the products of fermentation need not act as “hydrogen sinks,” in which the energy in the molecule is lost when they leave the cell; instead, the products of fermentation can be degraded further, during phase III of catabolism, and all the usable chemical energy of the nutrient can be transformed into ATP.
This section describes the manner in which the products obtained by the fragmentation of nutrients are oxidized (i.e., the manner in which hydrogen atoms or electrons are removed from them) and the manner in which these reducing equivalents react with oxygen, with concomitant formation of ATP.
The oxidation of molecular fragments
The oxidation of pyruvate
The oxidation of pyruvate involves the concerted action of several enzymes and coenzymes collectively called the pyruvate dehydrogenase complex; i.e., a multienzyme complex in which the substrates are passed consecutively from one enzyme to the next, and the product of the reaction catalyzed by the first enzyme immediately becomes the substrate for the second enzyme in the complex. The overall reaction is the formation of acetyl coenzyme A and carbon dioxide from pyruvate, with concomitant liberation of two reducing equivalents in the form of NADH + H+. The individual reactions that result in the formation of these end products are as follows.
Pyruvate first reacts with the coenzyme of pyruvic acid decarboxylase (enzyme 1), thiamine pyrophosphate (TPP); in addition to carbon dioxide a hydroxyethyl–TPP–enzyme complex (“active acetaldehyde”) is formed 【34】. Thiamine is vitamin B1; the biological role of TPP was first revealed by the inability of vitamin B1-deficient animals to oxidize pyruvate.
The hydroxyethyl moiety formed in 【34--> 】 is immediately transferred to one of the two sulfur atoms (S) of the coenzyme (6,8-dithio-n-octanoate or lipS2) of the second enzyme in the complex, dihydrolipoyl transacetylase (enzyme 2). The hydroxyethyl group attaches to lipS2 at one of its sulfur atoms, as shown in 【35-->
】 is immediately transferred to one of the two sulfur atoms (S) of the coenzyme (6,8-dithio-n-octanoate or lipS2) of the second enzyme in the complex, dihydrolipoyl transacetylase (enzyme 2). The hydroxyethyl group attaches to lipS2 at one of its sulfur atoms, as shown in 【35--> 】; the result is that coenzyme lipS2 is reduced and the hydroxyethyl moiety is oxidized.
】; the result is that coenzyme lipS2 is reduced and the hydroxyethyl moiety is oxidized.
 】 is immediately transferred to one of the two sulfur atoms (S) of the coenzyme (6,8-dithio-n-octanoate or lipS2) of the second enzyme in the complex, dihydrolipoyl transacetylase (enzyme 2). The hydroxyethyl group attaches to lipS2 at one of its sulfur atoms, as shown in 【35-->
】 is immediately transferred to one of the two sulfur atoms (S) of the coenzyme (6,8-dithio-n-octanoate or lipS2) of the second enzyme in the complex, dihydrolipoyl transacetylase (enzyme 2). The hydroxyethyl group attaches to lipS2 at one of its sulfur atoms, as shown in 【35--> 】; the result is that coenzyme lipS2 is reduced and the hydroxyethyl moiety is oxidized.
】; the result is that coenzyme lipS2 is reduced and the hydroxyethyl moiety is oxidized.
The acetyl group (CH3C∣=O) then is transferred to the sulfhydryl (−SH) group of coenzyme A, thereby completing the oxidation of pyruvate (reaction 【36--> 】).
】).
 】).
】).The coenzyme lipS2 that accepted the hydroxyethyl moiety in step 【35--> 】 of the sequence, now reduced, must be reoxidized before another molecule of pyruvate can be oxidized. The reoxidation of the coenzyme is achieved by the enzyme-catalyzed transfer of two reducing equivalents initially to the coenzyme flavin adenine dinucleotide (FAD) and thence to the NAD+ that is the first carrier in the so-called electron transport chain. The passage of two such reducing equivalents from reduced NAD+ to oxygen is accompanied by the formation of three molecules of ATP (see Biological energy transduction (metabolism)).
】 of the sequence, now reduced, must be reoxidized before another molecule of pyruvate can be oxidized. The reoxidation of the coenzyme is achieved by the enzyme-catalyzed transfer of two reducing equivalents initially to the coenzyme flavin adenine dinucleotide (FAD) and thence to the NAD+ that is the first carrier in the so-called electron transport chain. The passage of two such reducing equivalents from reduced NAD+ to oxygen is accompanied by the formation of three molecules of ATP (see Biological energy transduction (metabolism)).
 】 of the sequence, now reduced, must be reoxidized before another molecule of pyruvate can be oxidized. The reoxidation of the coenzyme is achieved by the enzyme-catalyzed transfer of two reducing equivalents initially to the coenzyme flavin adenine dinucleotide (FAD) and thence to the NAD+ that is the first carrier in the so-called electron transport chain. The passage of two such reducing equivalents from reduced NAD+ to oxygen is accompanied by the formation of three molecules of ATP (see Biological energy transduction (metabolism)).
】 of the sequence, now reduced, must be reoxidized before another molecule of pyruvate can be oxidized. The reoxidation of the coenzyme is achieved by the enzyme-catalyzed transfer of two reducing equivalents initially to the coenzyme flavin adenine dinucleotide (FAD) and thence to the NAD+ that is the first carrier in the so-called electron transport chain. The passage of two such reducing equivalents from reduced NAD+ to oxygen is accompanied by the formation of three molecules of ATP (see Biological energy transduction (metabolism)).
The overall reaction may be written as shown in 【37-->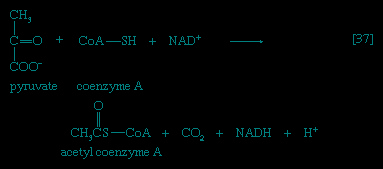 】, in which pyruvate reacts with coenzyme A in the presence of TPP and lipS2 to form acetyl coenzyme A and carbon dioxide, and to liberate two hydrogen atoms (in the form of NADH + H+) that can subsequently yield energy by the reduction of oxygen to water. The lipS2 reduced during this process is reoxidized in the presence of the enzyme lipoyl dehydrogenase, with the concomitant reduction of NAD+.
】, in which pyruvate reacts with coenzyme A in the presence of TPP and lipS2 to form acetyl coenzyme A and carbon dioxide, and to liberate two hydrogen atoms (in the form of NADH + H+) that can subsequently yield energy by the reduction of oxygen to water. The lipS2 reduced during this process is reoxidized in the presence of the enzyme lipoyl dehydrogenase, with the concomitant reduction of NAD+.
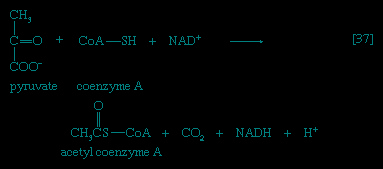 】, in which pyruvate reacts with coenzyme A in the presence of TPP and lipS2 to form acetyl coenzyme A and carbon dioxide, and to liberate two hydrogen atoms (in the form of NADH + H+) that can subsequently yield energy by the reduction of oxygen to water. The lipS2 reduced during this process is reoxidized in the presence of the enzyme lipoyl dehydrogenase, with the concomitant reduction of NAD+.
】, in which pyruvate reacts with coenzyme A in the presence of TPP and lipS2 to form acetyl coenzyme A and carbon dioxide, and to liberate two hydrogen atoms (in the form of NADH + H+) that can subsequently yield energy by the reduction of oxygen to water. The lipS2 reduced during this process is reoxidized in the presence of the enzyme lipoyl dehydrogenase, with the concomitant reduction of NAD+.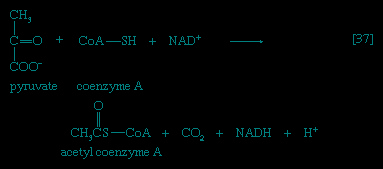
The tricarboxylic acid (TCA (tricarboxylic acid cycle)) cycle

 Special Comp-->
Special Comp--> Acetyl coenzyme A arises not only from the oxidation of pyruvate but also from that of fats (Figure 5-->
Acetyl coenzyme A arises not only from the oxidation of pyruvate but also from that of fats (Figure 5--> ) and many of the amino acids comprising proteins (Figure 2-->
) and many of the amino acids comprising proteins (Figure 2--> ); the sequence of enzyme-catalyzed steps that effects the total combustion of the acetyl moiety of the coenzyme represents the terminal oxidative pathway for virtually all food materials. The balance of the overall reaction of the TCA cycle 【37a-->
); the sequence of enzyme-catalyzed steps that effects the total combustion of the acetyl moiety of the coenzyme represents the terminal oxidative pathway for virtually all food materials. The balance of the overall reaction of the TCA cycle 【37a--> 】 is that three molecules of water react with acetyl coenzyme A to form carbon dioxide, coenzyme A, and reducing equivalents. The oxidation by oxygen of the reducing equivalents is accompanied by the conservation (as ATP) of most of the energy of the food ingested by aerobic organisms.
】 is that three molecules of water react with acetyl coenzyme A to form carbon dioxide, coenzyme A, and reducing equivalents. The oxidation by oxygen of the reducing equivalents is accompanied by the conservation (as ATP) of most of the energy of the food ingested by aerobic organisms.
Formation of coenzyme A, carbon dioxide, and reducing equivalent
The relative complexity and number of chemical events that constitute the TCA cycle, and their location as components of spatially determined structures such as cell membranes in microorganisms and mitochondria in plants and higher animals, reflect the problems involved chemically in “dismembering” a compound having only two carbon atoms and releasing in a controlled and stepwise manner the reducing equivalents ultimately to be passed to oxygen. These problems have been overcome by the simple but effective device of initially combining the two-carbon compound with a four-carbon acceptor; it is much less difficult chemically to dismember and oxidize a compound having six carbon atoms.
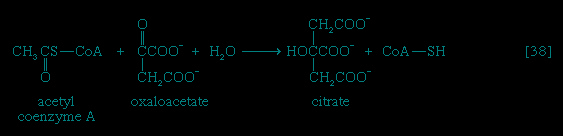
In the TCA cycle, acetyl coenzyme A initially reacts with oxaloacetate to yield citrate and to liberate coenzyme A. This reaction 【38-->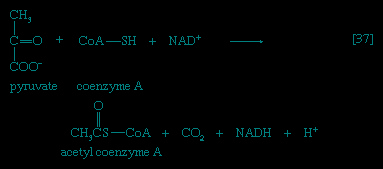 】 is catalyzed by citrate synthase. (As mentioned above, many of the compounds in living cells that take part in metabolic pathways exist as charged moieties, or anions, and are named as such.) Citrate undergoes isomerization (i.e., a rearrangement of certain atoms comprising the molecule) to form isocitrate 【39-->
】 is catalyzed by citrate synthase. (As mentioned above, many of the compounds in living cells that take part in metabolic pathways exist as charged moieties, or anions, and are named as such.) Citrate undergoes isomerization (i.e., a rearrangement of certain atoms comprising the molecule) to form isocitrate 【39-->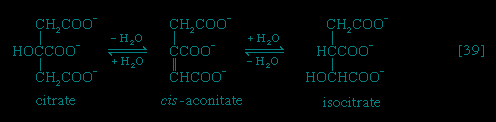 】. The reaction involves first the removal of the elements of water from citrate to form cis-aconitate, and then the re-addition of water to cis-aconitate in such a way that isocitrate is formed. It is probable that all three reactants—citrate, cis-aconitate, and isocitrate—remain closely associated with aconitase, the enzyme that catalyzes the isomerization process, and that most of the cis-aconitate is not released from the enzyme surface but is immediately converted to isocitrate.
】. The reaction involves first the removal of the elements of water from citrate to form cis-aconitate, and then the re-addition of water to cis-aconitate in such a way that isocitrate is formed. It is probable that all three reactants—citrate, cis-aconitate, and isocitrate—remain closely associated with aconitase, the enzyme that catalyzes the isomerization process, and that most of the cis-aconitate is not released from the enzyme surface but is immediately converted to isocitrate.
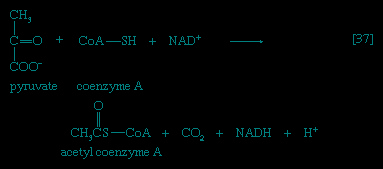 】 is catalyzed by citrate synthase. (As mentioned above, many of the compounds in living cells that take part in metabolic pathways exist as charged moieties, or anions, and are named as such.) Citrate undergoes isomerization (i.e., a rearrangement of certain atoms comprising the molecule) to form isocitrate 【39-->
】 is catalyzed by citrate synthase. (As mentioned above, many of the compounds in living cells that take part in metabolic pathways exist as charged moieties, or anions, and are named as such.) Citrate undergoes isomerization (i.e., a rearrangement of certain atoms comprising the molecule) to form isocitrate 【39-->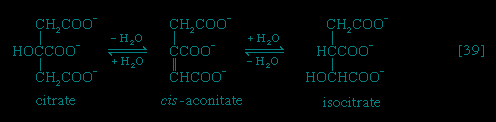 】. The reaction involves first the removal of the elements of water from citrate to form cis-aconitate, and then the re-addition of water to cis-aconitate in such a way that isocitrate is formed. It is probable that all three reactants—citrate, cis-aconitate, and isocitrate—remain closely associated with aconitase, the enzyme that catalyzes the isomerization process, and that most of the cis-aconitate is not released from the enzyme surface but is immediately converted to isocitrate.
】. The reaction involves first the removal of the elements of water from citrate to form cis-aconitate, and then the re-addition of water to cis-aconitate in such a way that isocitrate is formed. It is probable that all three reactants—citrate, cis-aconitate, and isocitrate—remain closely associated with aconitase, the enzyme that catalyzes the isomerization process, and that most of the cis-aconitate is not released from the enzyme surface but is immediately converted to isocitrate.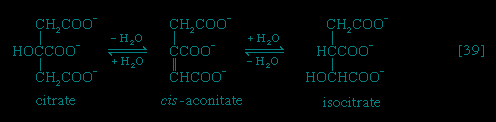
Isocitrate is oxidized—i.e., hydrogen is removed—to form oxalosuccinate; the two hydrogen atoms are usually transferred to NAD+, thus forming reduced NAD+ 【40-->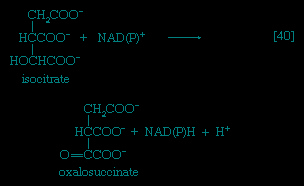 】; in some microorganisms, and during the biosynthesis of glutamate in the cytoplasm of animal cells, however, the
】; in some microorganisms, and during the biosynthesis of glutamate in the cytoplasm of animal cells, however, the
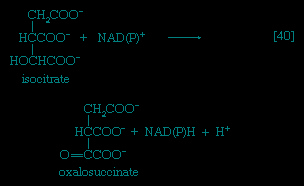 】; in some microorganisms, and during the biosynthesis of glutamate in the cytoplasm of animal cells, however, the
】; in some microorganisms, and during the biosynthesis of glutamate in the cytoplasm of animal cells, however, the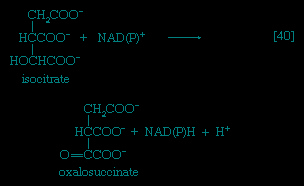
hydrogen atoms may also be accepted by NADP+. Thus the enzyme controlling this reaction, isocitrate dehydrogenase, differs in specificity for the coenzymes; various forms occur not only in different organisms but even within the same cell. In 【40-->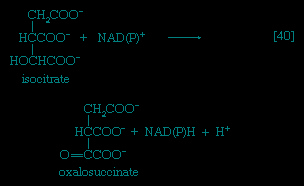 】 NAD(P)+ indicates that either NAD+ or NADP+ can act as a hydrogen acceptor.
】 NAD(P)+ indicates that either NAD+ or NADP+ can act as a hydrogen acceptor.
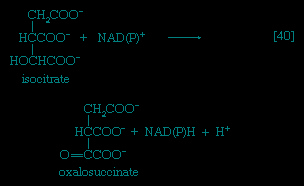 】 NAD(P)+ indicates that either NAD+ or NADP+ can act as a hydrogen acceptor.
】 NAD(P)+ indicates that either NAD+ or NADP+ can act as a hydrogen acceptor.The position of the carboxylate (−COO-) that is “sandwiched” in the middle of the oxalosuccinate molecule renders it very unstable; as a result, the carbon of this group is lost as carbon dioxide (note the dotted rectangle) in a reaction 【41-->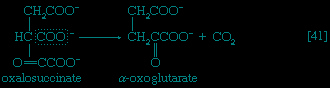 】 that can occur spontaneously but may be further accelerated by an enzyme.
】 that can occur spontaneously but may be further accelerated by an enzyme.
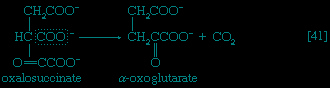 】 that can occur spontaneously but may be further accelerated by an enzyme.
】 that can occur spontaneously but may be further accelerated by an enzyme.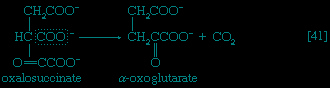
The five-carbon product of reaction 【41-->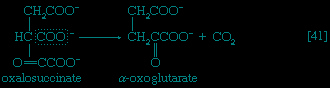 】, α-oxoglutarate, has chemical properties similar to pyruvate (free-acid forms of both are so-called α-oxoacids), and the chemical events involved in the oxidation of α-oxoglutarate are analogous to those already described for the oxidation of pyruvate (see reaction 【37-->
】, α-oxoglutarate, has chemical properties similar to pyruvate (free-acid forms of both are so-called α-oxoacids), and the chemical events involved in the oxidation of α-oxoglutarate are analogous to those already described for the oxidation of pyruvate (see reaction 【37-->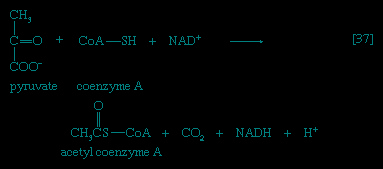 】). Reaction 【42-->
】). Reaction 【42-->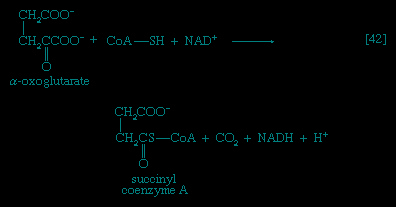 】 is effected by a multi-enzyme complex; TPP, lipS2 (6,8-dithio-n-octanoate), and coenzyme A are required as coenzymes. The products are carbon dioxide and succinyl coenzyme A. As was noted with reaction 【37-->
】 is effected by a multi-enzyme complex; TPP, lipS2 (6,8-dithio-n-octanoate), and coenzyme A are required as coenzymes. The products are carbon dioxide and succinyl coenzyme A. As was noted with reaction 【37-->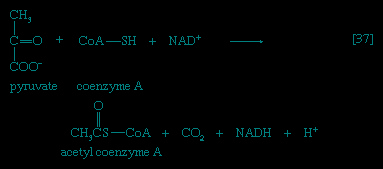 】, this oxidation of α-oxoglutarate results in the reduction of lipS2, which must be reoxidized. This is done by transfer of reducing equivalents to FAD and thence to NAD+. The resultant NADH + H+ is reoxidized by the passage of the electrons, ultimately, to oxygen, via the electron transport chain.
】, this oxidation of α-oxoglutarate results in the reduction of lipS2, which must be reoxidized. This is done by transfer of reducing equivalents to FAD and thence to NAD+. The resultant NADH + H+ is reoxidized by the passage of the electrons, ultimately, to oxygen, via the electron transport chain.
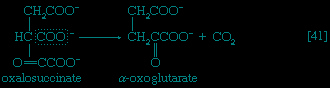 】, α-oxoglutarate, has chemical properties similar to pyruvate (free-acid forms of both are so-called α-oxoacids), and the chemical events involved in the oxidation of α-oxoglutarate are analogous to those already described for the oxidation of pyruvate (see reaction 【37-->
】, α-oxoglutarate, has chemical properties similar to pyruvate (free-acid forms of both are so-called α-oxoacids), and the chemical events involved in the oxidation of α-oxoglutarate are analogous to those already described for the oxidation of pyruvate (see reaction 【37-->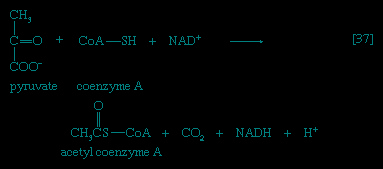 】). Reaction 【42-->
】). Reaction 【42--> 】 is effected by a multi-enzyme complex; TPP, lipS2 (6,8-dithio-n-octanoate), and coenzyme A are required as coenzymes. The products are carbon dioxide and succinyl coenzyme A. As was noted with reaction 【37-->
】 is effected by a multi-enzyme complex; TPP, lipS2 (6,8-dithio-n-octanoate), and coenzyme A are required as coenzymes. The products are carbon dioxide and succinyl coenzyme A. As was noted with reaction 【37-->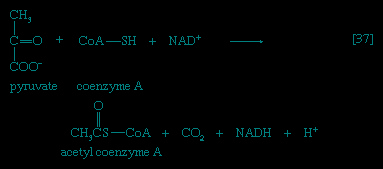 】, this oxidation of α-oxoglutarate results in the reduction of lipS2, which must be reoxidized. This is done by transfer of reducing equivalents to FAD and thence to NAD+. The resultant NADH + H+ is reoxidized by the passage of the electrons, ultimately, to oxygen, via the electron transport chain.
】, this oxidation of α-oxoglutarate results in the reduction of lipS2, which must be reoxidized. This is done by transfer of reducing equivalents to FAD and thence to NAD+. The resultant NADH + H+ is reoxidized by the passage of the electrons, ultimately, to oxygen, via the electron transport chain.
Unlike the acetyl coenzyme A produced from pyruvate in reaction 【37-->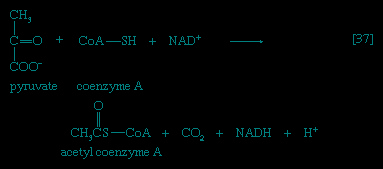 】, succinyl coenzyme A undergoes a phosphorolysis reaction—i.e., transfer of the succinyl moiety from coenzyme A to inorganic phosphate. The succinyl phosphate thus formed is not released from the enzyme surface; an unstable, high-energy compound called an acid anhydride, it transfers a high-energy phosphate to ADP, directly or via guanosine diphosphate (GDP) 【43-->
】, succinyl coenzyme A undergoes a phosphorolysis reaction—i.e., transfer of the succinyl moiety from coenzyme A to inorganic phosphate. The succinyl phosphate thus formed is not released from the enzyme surface; an unstable, high-energy compound called an acid anhydride, it transfers a high-energy phosphate to ADP, directly or via guanosine diphosphate (GDP) 【43--> 】.
】.
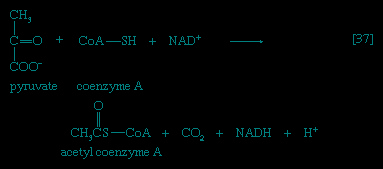 】, succinyl coenzyme A undergoes a phosphorolysis reaction—i.e., transfer of the succinyl moiety from coenzyme A to inorganic phosphate. The succinyl phosphate thus formed is not released from the enzyme surface; an unstable, high-energy compound called an acid anhydride, it transfers a high-energy phosphate to ADP, directly or via guanosine diphosphate (GDP) 【43-->
】, succinyl coenzyme A undergoes a phosphorolysis reaction—i.e., transfer of the succinyl moiety from coenzyme A to inorganic phosphate. The succinyl phosphate thus formed is not released from the enzyme surface; an unstable, high-energy compound called an acid anhydride, it transfers a high-energy phosphate to ADP, directly or via guanosine diphosphate (GDP) 【43--> 】.
】.
If guanosine triphosphate (GTP) forms, ATP can readily arise from it in an exchange involving ADP 【43a--> 】:
】:
 】:
】:
Regeneration of oxaloacetate
Special Comp--> Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->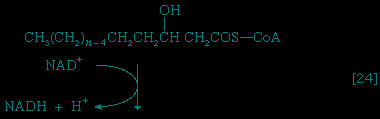
 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> The remainder of the reactions of the TCA cycle serve to regenerate the initial four-carbon acceptor of acetyl coenzyme A (oxaloacetate) from succinate, the process requiring in effect the oxidation of a methylene group (−CH2−) to a carbonyl group (−CO−), with concomitant release of 2 × 【2H】 reducing equivalents. It is therefore similar to, and is effected in like manner to, the oxidation of fatty acids (steps 【22-->
The remainder of the reactions of the TCA cycle serve to regenerate the initial four-carbon acceptor of acetyl coenzyme A (oxaloacetate) from succinate, the process requiring in effect the oxidation of a methylene group (−CH2−) to a carbonyl group (−CO−), with concomitant release of 2 × 【2H】 reducing equivalents. It is therefore similar to, and is effected in like manner to, the oxidation of fatty acids (steps 【22-->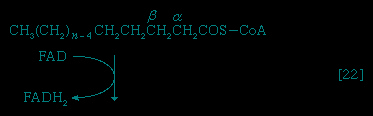 ,23-->
,23-->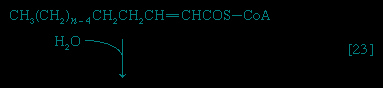 , and 24-->
, and 24-->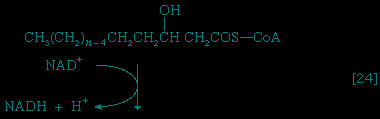 】; see Figure 5-->
】; see Figure 5--> ). As is the case with fatty acids, hydrogen atoms or electrons are initially removed from the succinate formed in 【43-->
). As is the case with fatty acids, hydrogen atoms or electrons are initially removed from the succinate formed in 【43--> 】 and are accepted by FAD; the reaction, catalyzed by succinate dehydrogenase 【44-->
】 and are accepted by FAD; the reaction, catalyzed by succinate dehydrogenase 【44--> 】, results in the formation of fumarate and reduced FAD.
】, results in the formation of fumarate and reduced FAD.
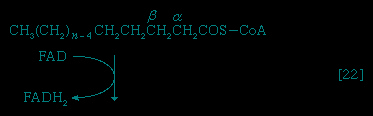 Special Comp-->
Special Comp-->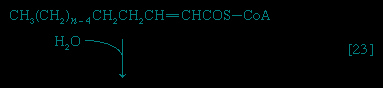 Special Comp-->
Special Comp-->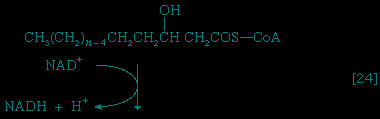
 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> The remainder of the reactions of the TCA cycle serve to regenerate the initial four-carbon acceptor of acetyl coenzyme A (oxaloacetate) from succinate, the process requiring in effect the oxidation of a methylene group (−CH2−) to a carbonyl group (−CO−), with concomitant release of 2 × 【2H】 reducing equivalents. It is therefore similar to, and is effected in like manner to, the oxidation of fatty acids (steps 【22-->
The remainder of the reactions of the TCA cycle serve to regenerate the initial four-carbon acceptor of acetyl coenzyme A (oxaloacetate) from succinate, the process requiring in effect the oxidation of a methylene group (−CH2−) to a carbonyl group (−CO−), with concomitant release of 2 × 【2H】 reducing equivalents. It is therefore similar to, and is effected in like manner to, the oxidation of fatty acids (steps 【22-->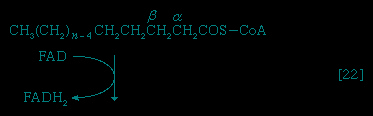 ,23-->
,23-->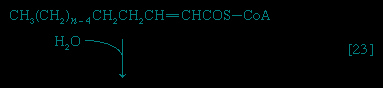 , and 24-->
, and 24-->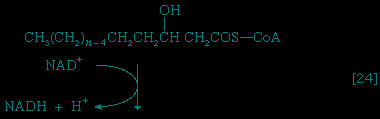 】; see Figure 5-->
】; see Figure 5--> ). As is the case with fatty acids, hydrogen atoms or electrons are initially removed from the succinate formed in 【43-->
). As is the case with fatty acids, hydrogen atoms or electrons are initially removed from the succinate formed in 【43--> 】 and are accepted by FAD; the reaction, catalyzed by succinate dehydrogenase 【44-->
】 and are accepted by FAD; the reaction, catalyzed by succinate dehydrogenase 【44--> 】, results in the formation of fumarate and reduced FAD.
】, results in the formation of fumarate and reduced FAD.
The elements of water are added across the double bond (−CH=CH−) of fumarate in a reaction catalyzed by fumarase 【45--> 】; this type of reaction also occurred in step 【39-->
】; this type of reaction also occurred in step 【39-->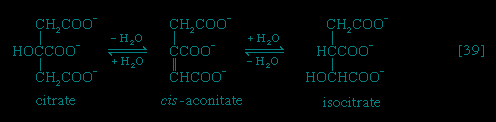 】 of the cycle. The product of reaction 【45-->
】 of the cycle. The product of reaction 【45--> 】 is malate.
】 is malate.
 】; this type of reaction also occurred in step 【39-->
】; this type of reaction also occurred in step 【39-->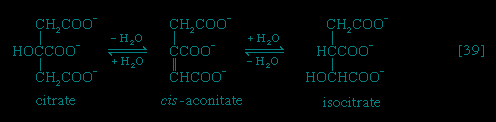 】 of the cycle. The product of reaction 【45-->
】 of the cycle. The product of reaction 【45--> 】 is malate.
】 is malate.
Malate can be oxidized to oxaloacetate by removal of two hydrogen atoms, which are accepted by NAD+. This type of reaction, catalyzed by malate dehydrogenase in reaction 【46-->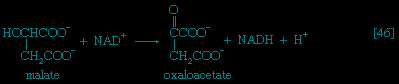 】, also occurred in step 【40-->
】, also occurred in step 【40-->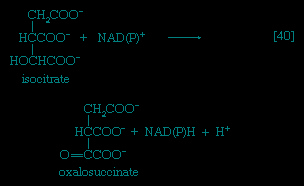 】 of the cycle. The formation of oxaloacetate completes the TCA cycle, which can now begin again with the formation of citrate 【38-->
】 of the cycle. The formation of oxaloacetate completes the TCA cycle, which can now begin again with the formation of citrate 【38--> 】.
】.
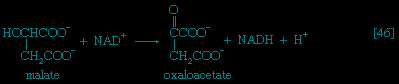 】, also occurred in step 【40-->
】, also occurred in step 【40-->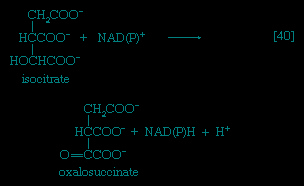 】 of the cycle. The formation of oxaloacetate completes the TCA cycle, which can now begin again with the formation of citrate 【38-->
】 of the cycle. The formation of oxaloacetate completes the TCA cycle, which can now begin again with the formation of citrate 【38--> 】.
】.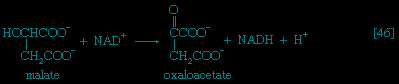
ATP yield of aerobic oxidation
The loss of the two molecules of carbon dioxide in steps 【41-->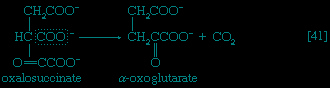 】 and 【42-->
】 and 【42--> 】 does not yield biologically useful energy. The substrate-linked formation of ATP accompanies step 【43-->
】 does not yield biologically useful energy. The substrate-linked formation of ATP accompanies step 【43--> 】, in which one molecule of of ATP, is formed during each turn of the cycle. The hydrogen ions and electrons that result from steps 【40-->
】, in which one molecule of of ATP, is formed during each turn of the cycle. The hydrogen ions and electrons that result from steps 【40-->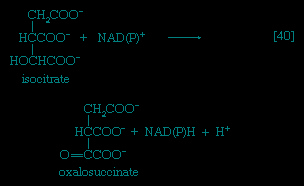 】, 【42-->
】, 【42--> 】, 【44-->
】, 【44--> 】, and 【46-->
】, and 【46-->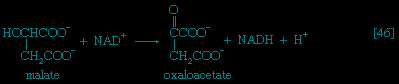 】 are passed down the chain of respiratory carriers to oxygen, with the concomitant formation of three molecules of ATP, per 【2H】, as NADH + H+ (see below). Similarly, the oxidation of the reduced FAD formed in 【44-->
】 are passed down the chain of respiratory carriers to oxygen, with the concomitant formation of three molecules of ATP, per 【2H】, as NADH + H+ (see below). Similarly, the oxidation of the reduced FAD formed in 【44--> 】 results in the formation of two ATP. Each turn of the cycle thus leads to the production of a total of 12 ATP. It will be recalled that the anaerobic fragmentation of glucose to two molecules of pyruvate yielded two ATP; the aerobic oxidation via the TCA cycle of two molecules of pyruvate thus makes available to the cell at least 15 times more ATP per molecule of glucose catabolized than is produced anaerobically. If, in addition, the 2 × 【NADH + H+】 generated per glucose in reaction 【6-->
】 results in the formation of two ATP. Each turn of the cycle thus leads to the production of a total of 12 ATP. It will be recalled that the anaerobic fragmentation of glucose to two molecules of pyruvate yielded two ATP; the aerobic oxidation via the TCA cycle of two molecules of pyruvate thus makes available to the cell at least 15 times more ATP per molecule of glucose catabolized than is produced anaerobically. If, in addition, the 2 × 【NADH + H+】 generated per glucose in reaction 【6--> 】 are passed on to oxygen, a further six ATP are generated. The advantage to living organisms is to be able to respire rather than merely to ferment.
】 are passed on to oxygen, a further six ATP are generated. The advantage to living organisms is to be able to respire rather than merely to ferment.
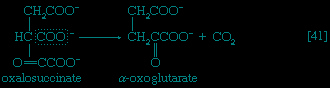 】 and 【42-->
】 and 【42--> 】 does not yield biologically useful energy. The substrate-linked formation of ATP accompanies step 【43-->
】 does not yield biologically useful energy. The substrate-linked formation of ATP accompanies step 【43--> 】, in which one molecule of of ATP, is formed during each turn of the cycle. The hydrogen ions and electrons that result from steps 【40-->
】, in which one molecule of of ATP, is formed during each turn of the cycle. The hydrogen ions and electrons that result from steps 【40-->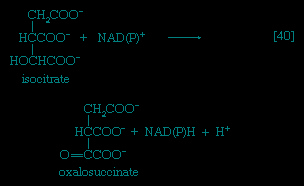 】, 【42-->
】, 【42--> 】, 【44-->
】, 【44--> 】, and 【46-->
】, and 【46-->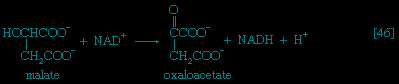 】 are passed down the chain of respiratory carriers to oxygen, with the concomitant formation of three molecules of ATP, per 【2H】, as NADH + H+ (see below). Similarly, the oxidation of the reduced FAD formed in 【44-->
】 are passed down the chain of respiratory carriers to oxygen, with the concomitant formation of three molecules of ATP, per 【2H】, as NADH + H+ (see below). Similarly, the oxidation of the reduced FAD formed in 【44--> 】 results in the formation of two ATP. Each turn of the cycle thus leads to the production of a total of 12 ATP. It will be recalled that the anaerobic fragmentation of glucose to two molecules of pyruvate yielded two ATP; the aerobic oxidation via the TCA cycle of two molecules of pyruvate thus makes available to the cell at least 15 times more ATP per molecule of glucose catabolized than is produced anaerobically. If, in addition, the 2 × 【NADH + H+】 generated per glucose in reaction 【6-->
】 results in the formation of two ATP. Each turn of the cycle thus leads to the production of a total of 12 ATP. It will be recalled that the anaerobic fragmentation of glucose to two molecules of pyruvate yielded two ATP; the aerobic oxidation via the TCA cycle of two molecules of pyruvate thus makes available to the cell at least 15 times more ATP per molecule of glucose catabolized than is produced anaerobically. If, in addition, the 2 × 【NADH + H+】 generated per glucose in reaction 【6--> 】 are passed on to oxygen, a further six ATP are generated. The advantage to living organisms is to be able to respire rather than merely to ferment.
】 are passed on to oxygen, a further six ATP are generated. The advantage to living organisms is to be able to respire rather than merely to ferment.Biological energy transduction
Adenosine triphosphate as the currency of energy exchange
When the terminal phosphate group is removed from ATP by hydrolysis, two negatively charged products are formed, ADP3- and HPO42- (a phosphate group) 【47-->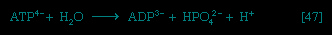 】.
】.
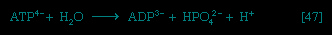 】.
】.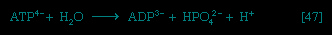
These products are electrically more stable than the parent molecule and do not readily recombine. The total free energy (G) of the products is much less than that of ATP; hence energy is liberated (i.e., the reaction is exergonic). The amount of energy liberated under strictly defined conditions is called the standard free energy change (ΔG′); this value for the hydrolysis of ATP is relatively high, at -8 kilocalories per mole. (One kilocalorie is the amount of heat required to raise the temperature of 1,000 grams of water one degree centigrade.) Conversely, the formation of ATP from ADP and inorganic phosphate (Pi) is an energy-requiring (i.e., endergonic) reaction with a standard free energy change of +8 kilocalories per mole.
The hydrolysis of the remaining phosphate-to-phosphate bond of ADP is also accompanied by a liberation of free energy (the standard free energy change is -6.5 kilocalories per mole); AMP hydrolysis liberates less energy (the standard free energy change is -2.2 kilocalories per mole).
 The free energy of hydrolysis of a compound thus is a measure of the difference in energy content between the starting substances (reactants) and the final substances (products). Adenosine triphosphate does not have the highest standard free energy of hydrolysis of all the naturally occurring phosphates but instead occupies a position at approximately the halfway point in a series of phosphate compounds with a wide range of standard free energies of hydrolysis. Compounds such as 1,3-diphosphoglycerate or phosphoenolpyruvate (PEP), which are above ATP on the scale (see Figure 6-->
The free energy of hydrolysis of a compound thus is a measure of the difference in energy content between the starting substances (reactants) and the final substances (products). Adenosine triphosphate does not have the highest standard free energy of hydrolysis of all the naturally occurring phosphates but instead occupies a position at approximately the halfway point in a series of phosphate compounds with a wide range of standard free energies of hydrolysis. Compounds such as 1,3-diphosphoglycerate or phosphoenolpyruvate (PEP), which are above ATP on the scale (see Figure 6--> ), have large negative ΔG′ values on hydrolysis and are often called high-energy phosphates; they are said to exhibit a high phosphate group transfer potential because they have a tendency to lose their phosphate groups. Compounds such as glucose 6-phosphate or fructose 6-phosphate, which are below ATP on the scale because they have smaller negative ΔG′ values on hydrolysis, have a tendency to hold on to their phosphate groups and thus act as low-energy phosphate acceptors.
), have large negative ΔG′ values on hydrolysis and are often called high-energy phosphates; they are said to exhibit a high phosphate group transfer potential because they have a tendency to lose their phosphate groups. Compounds such as glucose 6-phosphate or fructose 6-phosphate, which are below ATP on the scale because they have smaller negative ΔG′ values on hydrolysis, have a tendency to hold on to their phosphate groups and thus act as low-energy phosphate acceptors.Both ATP and ADP act as intermediate carriers for the transfer of phosphate groups (which are more precisely called phosphoryl groups) and hence of energy, from compounds lying above ATP to those lying beneath it. Thus, in glycolysis, ADP acts as an acceptor of a phosphate group during the synthesis of ATP from PEP (see reaction 【10】), and ATP functions as a donor of a phosphate group during the formation of fructose 1,6-diphosphate from fructose 6-phosphate (see reaction 【3--> 】).
】).
 】).
】).The first step in glycolysis, the formation of glucose 6-phosphate (G6P), illustrates how an energetically unfavourable reaction may become feasible under intracellular conditions by coupling it to ATP.


Reaction 【48--> 】 has a positive ΔG′ value, indicating that the reaction tends to proceed in the reverse direction. It is therefore necessary to use the standard free energy generated by the breaking of the first phosphate bond in ATP (reaction 【48a-->
】 has a positive ΔG′ value, indicating that the reaction tends to proceed in the reverse direction. It is therefore necessary to use the standard free energy generated by the breaking of the first phosphate bond in ATP (reaction 【48a--> 】), which is -7.3 kilocalories per mole, to move reaction 【48-->
】), which is -7.3 kilocalories per mole, to move reaction 【48-->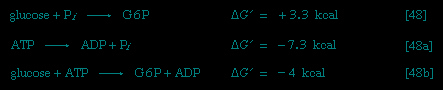 】 in the forward direction. Combining these reactions and their standard free energies gives reaction 【48b-->
】 in the forward direction. Combining these reactions and their standard free energies gives reaction 【48b--> 】 and a standard free energy value of -4 kilocalories per mole, indicating that the reaction will proceed in the forward direction. There are many intracellular reactions in which the formation of ADP or AMP from ATP provides energy for otherwise unfavourable biosyntheses. Some cellular reactions use equivalent phosphorylated analogues of ATP, for example, guanosine triphosphate (GTP) for protein synthesis.
】 and a standard free energy value of -4 kilocalories per mole, indicating that the reaction will proceed in the forward direction. There are many intracellular reactions in which the formation of ADP or AMP from ATP provides energy for otherwise unfavourable biosyntheses. Some cellular reactions use equivalent phosphorylated analogues of ATP, for example, guanosine triphosphate (GTP) for protein synthesis.
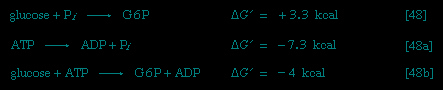 】 has a positive ΔG′ value, indicating that the reaction tends to proceed in the reverse direction. It is therefore necessary to use the standard free energy generated by the breaking of the first phosphate bond in ATP (reaction 【48a-->
】 has a positive ΔG′ value, indicating that the reaction tends to proceed in the reverse direction. It is therefore necessary to use the standard free energy generated by the breaking of the first phosphate bond in ATP (reaction 【48a--> 】), which is -7.3 kilocalories per mole, to move reaction 【48-->
】), which is -7.3 kilocalories per mole, to move reaction 【48-->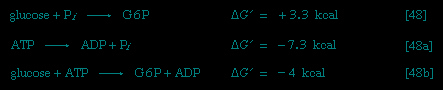 】 in the forward direction. Combining these reactions and their standard free energies gives reaction 【48b-->
】 in the forward direction. Combining these reactions and their standard free energies gives reaction 【48b--> 】 and a standard free energy value of -4 kilocalories per mole, indicating that the reaction will proceed in the forward direction. There are many intracellular reactions in which the formation of ADP or AMP from ATP provides energy for otherwise unfavourable biosyntheses. Some cellular reactions use equivalent phosphorylated analogues of ATP, for example, guanosine triphosphate (GTP) for protein synthesis.
】 and a standard free energy value of -4 kilocalories per mole, indicating that the reaction will proceed in the forward direction. There are many intracellular reactions in which the formation of ADP or AMP from ATP provides energy for otherwise unfavourable biosyntheses. Some cellular reactions use equivalent phosphorylated analogues of ATP, for example, guanosine triphosphate (GTP) for protein synthesis.The function of ATP as a common intermediate of energy transfer during anabolism is further dealt with below (see The biosynthesis of cell components (metabolism)). In certain specialized cells or tissues the chemical energy of ATP is used to perform work other than the chemical work of anabolism; (anabolism) for example, mechanical work—such as muscular contraction, or the movement of contractile structures called cilia and flagella, which are responsible for the motility of many small organisms. The performance of osmotic work also requires ATP; e.g., the transport of ions or metabolites through membranes against a concentration gradient, a process that is basically responsible for many physiological functions, including nerve conduction, the secretion of hydrochloric acid in the stomach, and the removal of water from the kidneys.
Energy conservation
The amount of ATP in a cell is limited, and it must be replaced continually to maintain repair and growth. This is achieved by using the energy liberated during the oxidative stages of catabolism to synthesize ATP from ADP and phosphate. The synthesis of ATP linked to catabolism occurs by two distinct mechanisms: substrate-level phosphorylation and oxidative, or respiratory-chain, phosphorylation. Oxidative phosphorylation is the major method of energy conservation under aerobic conditions in all nonphotosynthetic cells.
Substrate-level phosphorylation
In substrate-level phosphorylation a phosphoryl group is transferred from an energy-rich donor (e.g., 1,3-diphosphoglycerate) to ADP to yield a molecule of ATP. This type of ATP synthesis (see reactions 【7--> 】, 【10-->
】, 【10--> 】, and 【43-->
】, and 【43--> 】) does not require molecular oxygen (O2), although it is frequently, but not always, preceded by an oxidation (i.e., dehydrogenation) reaction. Substrate-level phosphorylation is the major method of energy conservation in oxygen-depleted tissues and during fermentative growth of microorganisms.
】) does not require molecular oxygen (O2), although it is frequently, but not always, preceded by an oxidation (i.e., dehydrogenation) reaction. Substrate-level phosphorylation is the major method of energy conservation in oxygen-depleted tissues and during fermentative growth of microorganisms.
 】, 【10-->
】, 【10--> 】, and 【43-->
】, and 【43--> 】) does not require molecular oxygen (O2), although it is frequently, but not always, preceded by an oxidation (i.e., dehydrogenation) reaction. Substrate-level phosphorylation is the major method of energy conservation in oxygen-depleted tissues and during fermentative growth of microorganisms.
】) does not require molecular oxygen (O2), although it is frequently, but not always, preceded by an oxidation (i.e., dehydrogenation) reaction. Substrate-level phosphorylation is the major method of energy conservation in oxygen-depleted tissues and during fermentative growth of microorganisms.Oxidative, or respiratory-chain, phosphorylation
In oxidative phosphorylation the oxidation of catabolic intermediates by molecular oxygen occurs via a highly ordered series of substances that act as hydrogen and electron carriers. They constitute the electron transfer system, or respiratory chain. In most animals, plants, and fungi, the electron transfer system is fixed in the membranes of mitochondria; in bacteria (which have no mitochondria) this system is incorporated into the plasma membrane. Sufficient free energy is released to allow the synthesis of ATP by a process described below. First, however, it is necessary to consider the nature of the respiratory chain.
The nature of the respiratory chain
Four types of hydrogen or electron carriers are known to participate in the respiratory chain, in which they serve to transfer two reducing equivalents (2H) from reduced substrate (AH2) to molecular oxygen (see reaction 【49--> 】); the products are the oxidized substrate (A) and water (H2O).
】); the products are the oxidized substrate (A) and water (H2O).
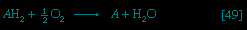 】); the products are the oxidized substrate (A) and water (H2O).
】); the products are the oxidized substrate (A) and water (H2O).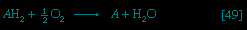
The carriers are NAD+ and, less frequently, NADP+; the flavoproteins FAD and FMN (flavin mononucleotide); ubiquinone (or coenzyme Q); and several types of cytochromes. Each carrier has an oxidized and reduced form (e.g., FAD and FADH2, respectively), the two forms constituting an oxidation-reduction (oxidation–reduction reaction), or redox, couple. Within the respiratory chain each redox couple undergoes cyclic oxidation-reduction—i.e., the oxidized component of the couple accepts reducing equivalents from either a substrate or a reduced carrier preceding it in the series and in turn donates these reducing equivalents to the next oxidized carrier in the sequence. Reducing equivalents are thus transferred from substrates to molecular oxygen by a number of sequential redox reactions.
Most oxidizable catabolic intermediates initially undergo a dehydrogenation reaction, during which a dehydrogenase enzyme transfers the equivalent of a hydride ion (H+ + 2e-, with e- representing an electron) to its coenzyme, either NAD+ or NADP+. The reduced NAD+ (or NADP+) thus produced (usually written as NADH + H+ or NADPH + H+) diffuses to the membrane-bound respiratory chain to be oxidized by an enzyme known as NADH dehydrogenase; the enzyme has as its coenzyme FMN. There is no corresponding NADPH dehydrogenase in mammalian mitochondria; instead, the reducing equivalents of NADPH + H+ are transferred to NAD+ in a reaction catalyzed by a transhydrogenase enzyme, with the products being reduced NADH + H+ and NADP+. A few substrates (e.g., acyl coenzyme A and succinate; see reactions 【22-->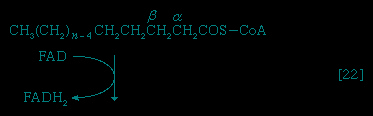 】 and 【44-->
】 and 【44--> 】) bypass this reaction and instead undergo immediate dehydrogenation by specific membrane-bound dehydrogenase enzymes. During the reaction, the coenzyme FAD accepts two hydrogen atoms and two electrons (2H + 2e-). The reduced flavoproteins (i.e., FMNH2 and FADH2) donate their two hydrogen atoms to the lipid carrier ubiquinone, which is thus reduced.
】) bypass this reaction and instead undergo immediate dehydrogenation by specific membrane-bound dehydrogenase enzymes. During the reaction, the coenzyme FAD accepts two hydrogen atoms and two electrons (2H + 2e-). The reduced flavoproteins (i.e., FMNH2 and FADH2) donate their two hydrogen atoms to the lipid carrier ubiquinone, which is thus reduced.
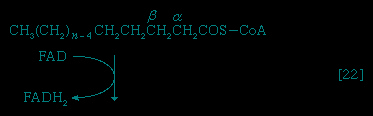 】 and 【44-->
】 and 【44--> 】) bypass this reaction and instead undergo immediate dehydrogenation by specific membrane-bound dehydrogenase enzymes. During the reaction, the coenzyme FAD accepts two hydrogen atoms and two electrons (2H + 2e-). The reduced flavoproteins (i.e., FMNH2 and FADH2) donate their two hydrogen atoms to the lipid carrier ubiquinone, which is thus reduced.
】) bypass this reaction and instead undergo immediate dehydrogenation by specific membrane-bound dehydrogenase enzymes. During the reaction, the coenzyme FAD accepts two hydrogen atoms and two electrons (2H + 2e-). The reduced flavoproteins (i.e., FMNH2 and FADH2) donate their two hydrogen atoms to the lipid carrier ubiquinone, which is thus reduced.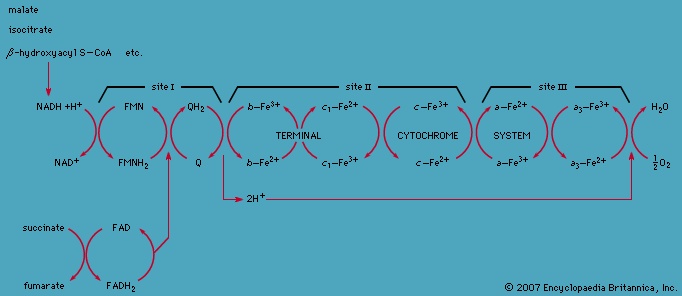 The fourth type of carrier, the cytochromes (cytochrome), consists of hemoproteins—i.e., proteins with a nonprotein component, or prosthetic group, called heme (or a derivative of heme), which is an iron-containing (iron) pigment molecule. The iron atom in the prosthetic group is able to carry one electron and oscillates between the oxidized, or ferric (Fe3+), and the reduced, or ferrous (Fe2+), forms. The five cytochromes present in the mammalian respiratory chain, designated cytochromes b, c1, c, a, and a3, act in sequence between ubiquinone and molecular oxygen. The terminal cytochrome of this sequence (a3, also known as cytochrome oxidase) is able to donate electrons to oxygen rather than to another electron carrier; a3 is also the site of action of two substances that inhibit the respiratory chain, potassium cyanide and carbon monoxide. Special Fe-S complexes play a role in the activity of NADH dehydrogenase and succinate dehydrogenase. The sequence of carriers, from substrates to oxygen, is shown schematically in Figure 7-->
The fourth type of carrier, the cytochromes (cytochrome), consists of hemoproteins—i.e., proteins with a nonprotein component, or prosthetic group, called heme (or a derivative of heme), which is an iron-containing (iron) pigment molecule. The iron atom in the prosthetic group is able to carry one electron and oscillates between the oxidized, or ferric (Fe3+), and the reduced, or ferrous (Fe2+), forms. The five cytochromes present in the mammalian respiratory chain, designated cytochromes b, c1, c, a, and a3, act in sequence between ubiquinone and molecular oxygen. The terminal cytochrome of this sequence (a3, also known as cytochrome oxidase) is able to donate electrons to oxygen rather than to another electron carrier; a3 is also the site of action of two substances that inhibit the respiratory chain, potassium cyanide and carbon monoxide. Special Fe-S complexes play a role in the activity of NADH dehydrogenase and succinate dehydrogenase. The sequence of carriers, from substrates to oxygen, is shown schematically in Figure 7--> .
.In each redox couple the reduced form has a tendency to lose reducing equivalents (i.e., to act as an electron or hydrogen donor); similarly, the oxidized form has a tendency to gain reducing equivalents (i.e., to act as an electron or hydrogen acceptor). The oxidation-reduction characteristics of each couple can be determined experimentally under well-defined, standard conditions. The value thus obtained is the standard oxidation-reduction (redox) potential (Eó). Values for respiratory chain carriers range from Eó = -320 millivolts (one millivolt = 0.001 volt) for NAD+/reduced NAD+ to Eó = +820 millivolts for 1/2O2/H2O; the values for intermediate carriers lie between. Reduced NAD+ is the most electronegative carrier, oxygen the most electropositive acceptor. During respiration reducing equivalents undergo stepwise transfer from the reduced form of the most electronegative carrier (reduced NAD+) to the oxidized form of the most electropositive couple (oxygen). Each step is accompanied by a decline in standard free energy (ΔG′) proportional to the difference in the standard redox potentials (ΔE0) of the two carriers involved.
Overall oxidation of reduced NAD+ by oxygen (ΔE0 = +1,140 millivolts) is accompanied by the liberation of free energy (ΔG′ = -52.4 kilocalories per mole); in theory this energy is sufficient to allow the synthesis of six or seven molecules of ATP. In the cell, however, this synthesis of ATP, called oxidative phosphorylation, proceeds with an efficiency of about 46 percent; thus only three molecules of ATP are produced per atom of oxygen consumed—this being the so-called P/2e-, P/O, or ADP/O ratio. The energy that is not conserved as ATP is lost as heat. The oxidation of succinate by molecular oxygen (ΔE0 = +790 millivolts), which is accompanied by a smaller liberation of free energy (ΔG′ = -36.5 kilocalories per mole), yields only two molecules of ATP per atom of oxygen consumed (P/O = 2).
ATP synthesis in mitochondria
In order to understand the mechanism by which the energy released during respiration is conserved as ATP, it is necessary to appreciate the structural features of mitochondria (mitochondrion). These are organelles in animal and plant cells in which oxidative phosphorylation takes place. There are many mitochondria in animal tissues; for example, in heart and skeletal muscle, which require large amounts of energy for mechanical work, in the pancreas, where there is biosynthesis, and in the kidney, where the process of excretion begins. Mitochondria have an outer membrane, which allows the passage of most small molecules and ions, and a highly folded inner membrane (crista), which does not even allow the passage of small ions and so maintains a closed space within the cell. The electron-transferring molecules of the respiratory chain and the enzymes responsible for ATP synthesis are located in and on this inner membrane, while the space inside (matrix) contains the enzymes of the TCA cycle (reactions 【34--> 】 to 【46-->
】 to 【46-->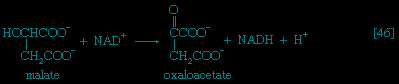 】; see also cell). The enzyme systems primarily responsible for the release and subsequent oxidation of reducing equivalents are thus closely related so that the reduced coenzymes formed during catabolism (NADH + H+ and FADH2) are available as substrates for respiration. The movement of most charged metabolites into the matrix space is mediated by special carrier proteins in the crista that catalyze exchange-diffusion (i.e., a one-for-one exchange). The oxidative phosphorylation systems of bacteria are similar in principle but show a greater diversity in the composition of their respiratory carriers.
】; see also cell). The enzyme systems primarily responsible for the release and subsequent oxidation of reducing equivalents are thus closely related so that the reduced coenzymes formed during catabolism (NADH + H+ and FADH2) are available as substrates for respiration. The movement of most charged metabolites into the matrix space is mediated by special carrier proteins in the crista that catalyze exchange-diffusion (i.e., a one-for-one exchange). The oxidative phosphorylation systems of bacteria are similar in principle but show a greater diversity in the composition of their respiratory carriers.
 】 to 【46-->
】 to 【46-->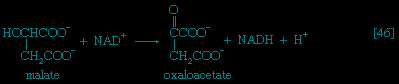 】; see also cell). The enzyme systems primarily responsible for the release and subsequent oxidation of reducing equivalents are thus closely related so that the reduced coenzymes formed during catabolism (NADH + H+ and FADH2) are available as substrates for respiration. The movement of most charged metabolites into the matrix space is mediated by special carrier proteins in the crista that catalyze exchange-diffusion (i.e., a one-for-one exchange). The oxidative phosphorylation systems of bacteria are similar in principle but show a greater diversity in the composition of their respiratory carriers.
】; see also cell). The enzyme systems primarily responsible for the release and subsequent oxidation of reducing equivalents are thus closely related so that the reduced coenzymes formed during catabolism (NADH + H+ and FADH2) are available as substrates for respiration. The movement of most charged metabolites into the matrix space is mediated by special carrier proteins in the crista that catalyze exchange-diffusion (i.e., a one-for-one exchange). The oxidative phosphorylation systems of bacteria are similar in principle but show a greater diversity in the composition of their respiratory carriers. The mechanism of ATP synthesis appears to be as follows. During the transfer of hydrogen atoms from FMNH2 or FADH2 to oxygen (Figure 7-->
The mechanism of ATP synthesis appears to be as follows. During the transfer of hydrogen atoms from FMNH2 or FADH2 to oxygen (Figure 7--> ), protons (H+ ions) are pumped across the crista from the inside of the mitochondrion to the outside. Thus, respiration generates an electrical potential (and in mitochondria a small pH gradient) across the membrane corresponding to 200 to 300 millivolts, and the chemical energy in the substrate is converted into electrical energy. Attached to the crista is a complex enzyme (ATP synthetase) that binds ATP, ADP, and Pi. It has nine polypeptide chain subunits of five different kinds in a cluster and a unit of at least three more membrane proteins composing the attachment point of ADP and Pi. This complex forms a specific proton pore in the membrane. When ADP and Pi are bound to ATP synthetase, the excess of protons (H+) that has formed outside of the mitochondria (an H+ gradient) moves back into the mitochondrion through the enzyme complex. The energy released is used to convert ADP and Pi to ATP. In this process, electrical energy is converted to chemical energy, and it is the supply of ADP that limits the rate of this process. The precise mechanism by which the ATP synthetase complex converts the energy stored in the electrical H+ gradient to the chemical bond energy in ATP is not well understood. The H+ gradient may power other endergonic (energy-requiring) processes besides ATP synthesis, such as the movement of bacterial cells and the transport of carbon substrates or ions.
), protons (H+ ions) are pumped across the crista from the inside of the mitochondrion to the outside. Thus, respiration generates an electrical potential (and in mitochondria a small pH gradient) across the membrane corresponding to 200 to 300 millivolts, and the chemical energy in the substrate is converted into electrical energy. Attached to the crista is a complex enzyme (ATP synthetase) that binds ATP, ADP, and Pi. It has nine polypeptide chain subunits of five different kinds in a cluster and a unit of at least three more membrane proteins composing the attachment point of ADP and Pi. This complex forms a specific proton pore in the membrane. When ADP and Pi are bound to ATP synthetase, the excess of protons (H+) that has formed outside of the mitochondria (an H+ gradient) moves back into the mitochondrion through the enzyme complex. The energy released is used to convert ADP and Pi to ATP. In this process, electrical energy is converted to chemical energy, and it is the supply of ADP that limits the rate of this process. The precise mechanism by which the ATP synthetase complex converts the energy stored in the electrical H+ gradient to the chemical bond energy in ATP is not well understood. The H+ gradient may power other endergonic (energy-requiring) processes besides ATP synthesis, such as the movement of bacterial cells and the transport of carbon substrates or ions.ATP formation during photosynthesis
Photosynthesis generates ATP by a mechanism that is similar in principle, if not in detail. The organelles responsible are different from mitochondria, but they also form membrane-bounded closed sacs (thylakoids) often arranged in stacks (grana). Solar energy splits two molecules of H2O into molecular oxygen (O2), four protons (H+), and four electrons.
This is the source of oxygen evolution, clearly visible as bubbles from underwater plants in bright sunshine. The process involves a chlorophyll molecule, P680, that changes its redox potential from +820 millivolts (in which there is a tendency to accept electrons) to about -680 millivolts (in which there is a tendency to lose electrons) upon excitation with light and acquisition of electrons. The electrons are subsequently passed along a series of carriers (plastoquinone, cytochromes b and f, and plastocyanin), analogous to the mitochondrial respiratory chain. This process pumps protons across the membrane from the outside of the thylakoid membrane to the inside. Protons (H+) do not move freely across the membrane although chloride ions (Cl-) do, creating a pH gradient. An ATP synthetase enzyme similar to that of the mitochondria is present, but on the outside of the thylakoid membrane. Passage of protons (H+) through it from inside to outside generates ATP.
Hence, a gradient of protons (H+) across the membrane is the high-energy intermediate for forming ATP in plant photosynthesis and in the respiration of all cells capable of passing reducing equivalents (hydrogen atoms or electrons) to electron acceptors.
The biosynthesis of cell components (anabolism)
The nature of biosynthesis
The stages of biosynthesis
The biosynthesis of cell components (anabolism) may be regarded as occurring in two main stages. In the first, intermediate compounds of the central routes of metabolism are diverted from further catabolism and are channeled into pathways that usually lead to the formation of the relatively small molecules that serve as the building blocks, or precursors, of macromolecules.
In the second stage of biosynthesis, the building blocks are combined to yield the macromolecules—proteins, nucleic acids, lipids, and polysaccharides—that make up the bulk of tissues and cellular components. In organisms with the appropriate genetic capability, for example, all of the amino acids can be synthesized from ammonia and intermediates of the main routes of carbohydrate fragmentation and oxidation. Such intermediates act also as precursors for the purines, the pyrimidines, and the pentose sugars that constitute DNA and for a number of types of RNA. The assembly of proteins necessitates the precise combination of specific amino acids in a highly ordered and controlled manner; this in turn involves the copying, or transcription, into RNA of specific parts of DNA (see below The synthesis of macromolecules: Nucleic acids and proteins (metabolism)). The first stage of biosynthesis thus requires the specificity normally required for the efficient functioning of sequences of enzyme-catalyzed reactions. The second stage also involves—directly for protein and nucleic acid synthesis, less directly for the synthesis of other macromolecules—the maintenance and expression of the biological information that specifies the identity of the cell, the tissue, and the organism.
Utilization of ATP
The two stages of biosynthesis—the formation of building blocks and their specific assembly into macromolecules—are energy-consuming processes and thus require ATP. Although the ATP is derived from catabolism, catabolism does not “drive” biosynthesis. As explained in the first section of this article, the occurrence of chemical reactions in the living cell is accompanied by a net decrease in free energy. Although biological growth and development result in the creation of ordered systems from less ordered ones and of complex systems from simpler ones, these events must occur at the expense of energy-yielding reactions. The overall coupled reactions are, on balance, still accompanied by a decrease in free energy and are thus essentially irreversible in the direction of biosynthesis. The total energy released from ATP, for example, is usually much greater than is needed for a particular biosynthetic step; thus, many of the reactions involved in biosynthesis release inorganic pyrophosphate (PPi) rather than phosphate (Pi) from ATP, and hence yield AMP rather than ADP. Since inorganic pyrophosphate readily undergoes virtually irreversible hydrolysis to two equivalents of inorganic phosphate (see 【21a-->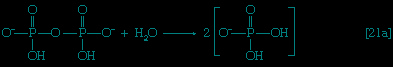 】), the creation of a new bond in the product of synthesis may be accompanied by the breaking of two high-energy bonds of ATP—although, in theory, one might have sufficed.
】), the creation of a new bond in the product of synthesis may be accompanied by the breaking of two high-energy bonds of ATP—although, in theory, one might have sufficed.
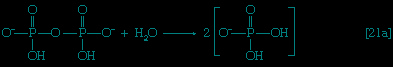 】), the creation of a new bond in the product of synthesis may be accompanied by the breaking of two high-energy bonds of ATP—although, in theory, one might have sufficed.
】), the creation of a new bond in the product of synthesis may be accompanied by the breaking of two high-energy bonds of ATP—although, in theory, one might have sufficed.The efficient utilization for anabolic processes of ATP and some intermediate compound formed during a catabolic reaction requires the cell to have simultaneously a milieu favourable for both ATP generation and consumption. Catabolism occurs readily only if sufficient ADP is available; hence, the concentration of ATP is low. On the other hand, biosynthesis requires a high level of ATP and consequently low levels of ADP and AMP. Suitable conditions for the simultaneous function of both processes are met in two ways. Biosynthetic reactions often take place in compartments within the cell different from those in which catabolism occurs; there is thus a physical separation of energy-requiring and energy-yielding processes. Furthermore, biosynthetic reactions are regulated independently of the mechanisms by which catabolism is controlled. Such independent control is made possible by the fact that catabolic and anabolic pathways are not identical; the pacemaker, or key, enzyme that controls the overall rate of a catabolic route usually does not play any role in the biosynthetic pathway of a compound. Similarly, the pacemaker enzymes of biosynthesis are not involved in catabolism. As discussed below (see Regulation of metabolism: Fine control: Energy state of the cell (metabolism)), catabolic pathways are often regulated by the relative amounts of ATP, ADP, and AMP in the cellular compartment in which the pacemaker enzymes are located; in general, ATP inhibits and ADP (or AMP) stimulates such enzymes. In contrast, many biosynthetic routes are regulated by the concentration of the end products of particular anabolic processes, so that the cell synthesizes only as much of these building blocks as it needs.
The supply of biosynthetic precursors

 When higher animals consume a mixed diet, sufficient quantities of compounds for both biosynthesis and energy supply are available. Carbohydrates yield intermediates of glycolysis and of the phosphogluconate pathway, which in turn yield acetyl coenzyme A (see Figure 4-->
When higher animals consume a mixed diet, sufficient quantities of compounds for both biosynthesis and energy supply are available. Carbohydrates yield intermediates of glycolysis and of the phosphogluconate pathway, which in turn yield acetyl coenzyme A (see Figure 4--> ); lipids yield glycolytic intermediates and acetyl coenzyme A (see Figure 2-->
); lipids yield glycolytic intermediates and acetyl coenzyme A (see Figure 2--> ); and many amino acids form intermediates of both the TCA cycle and glycolysis. Any intermediate withdrawn for biosynthesis can thus be readily replenished by the catabolism of further nutrients. This situation does not always hold, however. Microorganisms in particular can derive all of their carbon and energy requirements by utilizing a single carbon source. The sole carbon source may be a substance such as a carbohydrate or a fatty acid, or an intermediate of the TCA cycle (or a substance readily converted to one). In both cases, reactions ancillary to those discussed thus far must occur before the carbon source can be utilized.
); and many amino acids form intermediates of both the TCA cycle and glycolysis. Any intermediate withdrawn for biosynthesis can thus be readily replenished by the catabolism of further nutrients. This situation does not always hold, however. Microorganisms in particular can derive all of their carbon and energy requirements by utilizing a single carbon source. The sole carbon source may be a substance such as a carbohydrate or a fatty acid, or an intermediate of the TCA cycle (or a substance readily converted to one). In both cases, reactions ancillary to those discussed thus far must occur before the carbon source can be utilized.Anaplerotic routes
 Special Comp-->
Special Comp-->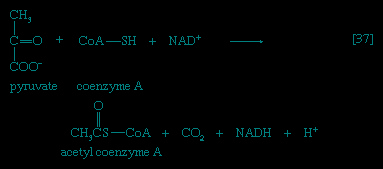 Special Comp-->
Special Comp-->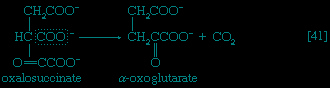 Special Comp-->
Special Comp-->
 Although the catabolism of carbohydrates can occur via a variety of routes (see Figure 4-->
Although the catabolism of carbohydrates can occur via a variety of routes (see Figure 4--> ), all give rise to pyruvate. During the catabolism of pyruvate, one carbon atom is lost as carbon dioxide and the remaining two form acetyl coenzyme A 【37-->
), all give rise to pyruvate. During the catabolism of pyruvate, one carbon atom is lost as carbon dioxide and the remaining two form acetyl coenzyme A 【37-->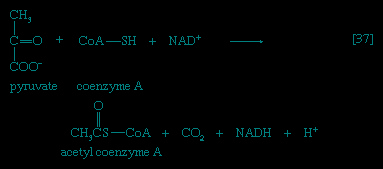 】; these two are involved in the TCA cycle (【41-->
】; these two are involved in the TCA cycle (【41-->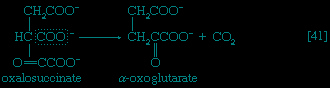 】 and 【42-->
】 and 【42--> 】). Because the TCA cycle is initiated by the condensation of acetyl coenzyme A with oxaloacetate, which is regenerated in each turn of the cycle, the removal of any intermediate from the cycle would cause the cycle to stop. Yet, as also indicated in Figure 4-->
】). Because the TCA cycle is initiated by the condensation of acetyl coenzyme A with oxaloacetate, which is regenerated in each turn of the cycle, the removal of any intermediate from the cycle would cause the cycle to stop. Yet, as also indicated in Figure 4--> , various essential cell components are derived from α-oxoglutarate, succinyl coenzyme A, and oxaloacetate, so that these compounds are, in fact, removed from the cycle. Microbial growth with a carbohydrate as the sole carbon source is thus possible only if a cellular process occurs that effects the net formation of some TCA cycle intermediate from an intermediate of carbohydrate catabolism. Such a process, which replenishes the TCA cycle, has been described as an anaplerotic reaction.
, various essential cell components are derived from α-oxoglutarate, succinyl coenzyme A, and oxaloacetate, so that these compounds are, in fact, removed from the cycle. Microbial growth with a carbohydrate as the sole carbon source is thus possible only if a cellular process occurs that effects the net formation of some TCA cycle intermediate from an intermediate of carbohydrate catabolism. Such a process, which replenishes the TCA cycle, has been described as an anaplerotic reaction.
The anaplerotic function may be carried out by either of two enzymes that catalyze the fixation of carbon dioxide onto a three-carbon compound, either pyruvate 【50--> 】 or phosphoenolpyruvate (PEP, 【50a-->
】 or phosphoenolpyruvate (PEP, 【50a-->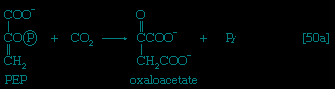 】) to form oxaloacetate, which has four carbon atoms. Both reactions require energy. In 【50-->
】) to form oxaloacetate, which has four carbon atoms. Both reactions require energy. In 【50--> 】 it is supplied by the cleavage of ATP to ADP and inorganic phosphate (Pi); in 【50a-->
】 it is supplied by the cleavage of ATP to ADP and inorganic phosphate (Pi); in 【50a--> 】 it is supplied by the release of the high-energy phosphate of PEP as inorganic phosphate. Pyruvate serves as a carbon dioxide acceptor not only in many bacteria and fungi but also in the livers and kidneys of higher organisms, including man; PEP serves as the carbon dioxide acceptor in many bacteria, such as those that inhabit the gut.
】 it is supplied by the release of the high-energy phosphate of PEP as inorganic phosphate. Pyruvate serves as a carbon dioxide acceptor not only in many bacteria and fungi but also in the livers and kidneys of higher organisms, including man; PEP serves as the carbon dioxide acceptor in many bacteria, such as those that inhabit the gut.
 】 or phosphoenolpyruvate (PEP, 【50a-->
】 or phosphoenolpyruvate (PEP, 【50a--> 】) to form oxaloacetate, which has four carbon atoms. Both reactions require energy. In 【50-->
】) to form oxaloacetate, which has four carbon atoms. Both reactions require energy. In 【50--> 】 it is supplied by the cleavage of ATP to ADP and inorganic phosphate (Pi); in 【50a-->
】 it is supplied by the cleavage of ATP to ADP and inorganic phosphate (Pi); in 【50a--> 】 it is supplied by the release of the high-energy phosphate of PEP as inorganic phosphate. Pyruvate serves as a carbon dioxide acceptor not only in many bacteria and fungi but also in the livers and kidneys of higher organisms, including man; PEP serves as the carbon dioxide acceptor in many bacteria, such as those that inhabit the gut.
】 it is supplied by the release of the high-energy phosphate of PEP as inorganic phosphate. Pyruvate serves as a carbon dioxide acceptor not only in many bacteria and fungi but also in the livers and kidneys of higher organisms, including man; PEP serves as the carbon dioxide acceptor in many bacteria, such as those that inhabit the gut.
Unlike higher organisms, many bacteria and fungi can grow on acetate or compounds such as ethanol or a fatty acid that can be catabolized to acetyl coenzyme A. Under these conditions, the net formation of TCA cycle intermediates proceeds in one of two ways. In obligate anaerobic bacteria, pyruvate can be formed from acetyl coenzyme A and carbon dioxide 【51-->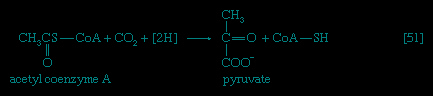 】; reducing equivalents 【2H】 are necessary for the reaction. The pyruvate so formed can then react via either step 【50-->
】; reducing equivalents 【2H】 are necessary for the reaction. The pyruvate so formed can then react via either step 【50--> 】 or 【50a-->
】 or 【50a--> 】.
】.
 】; reducing equivalents 【2H】 are necessary for the reaction. The pyruvate so formed can then react via either step 【50-->
】; reducing equivalents 【2H】 are necessary for the reaction. The pyruvate so formed can then react via either step 【50--> 】 or 【50a-->
】 or 【50a--> 】.
】.
Special Comp-->
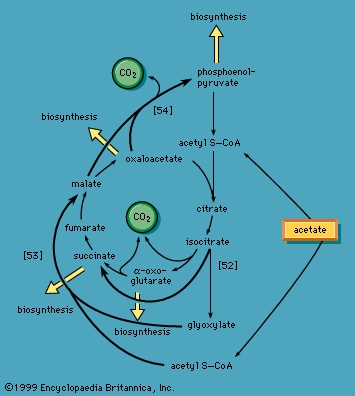 Special Comp-->
Special Comp-->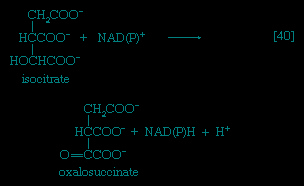 Special Comp-->
Special Comp-->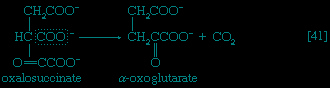 Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->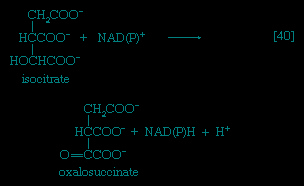 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->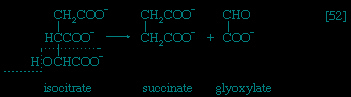 Reaction 【51-->
Reaction 【51--> 】 does not occur in facultative anaerobic organisms or in strict aerobes, however. Instead, in these organisms two molecules of acetyl coenzyme A give rise to the net synthesis of a four-carbon intermediate of the TCA cycle via a route known as the glyoxylate cycle. In this route (Figure 8-->
】 does not occur in facultative anaerobic organisms or in strict aerobes, however. Instead, in these organisms two molecules of acetyl coenzyme A give rise to the net synthesis of a four-carbon intermediate of the TCA cycle via a route known as the glyoxylate cycle. In this route (Figure 8--> ), the steps of the TCA cycle that lead to the loss of carbon dioxide (see 【40-->
), the steps of the TCA cycle that lead to the loss of carbon dioxide (see 【40-->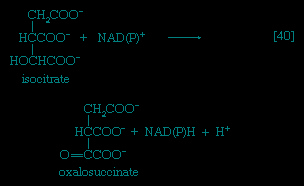 】, 【41-->
】, 【41-->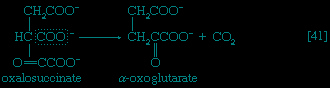 】, and 【42-->
】, and 【42--> 】) are bypassed. Instead of being oxidized to oxalosuccinate, as occurs in 【40-->
】) are bypassed. Instead of being oxidized to oxalosuccinate, as occurs in 【40-->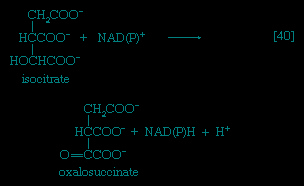 】, isocitrate is split by isocitrate lyase 【52-->
】, isocitrate is split by isocitrate lyase 【52-->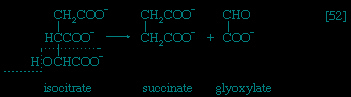 】 in a reaction similar to that of reactions 【4-->
】 in a reaction similar to that of reactions 【4--> 】 and 【15-->
】 and 【15--> 】 of carbohydrate fragmentation. The dotted line in 【52-->
】 of carbohydrate fragmentation. The dotted line in 【52-->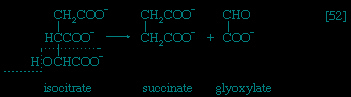 】 indicates the way in which isocitrate is split. The products are
】 indicates the way in which isocitrate is split. The products are

 Special Comp-->
Special Comp-->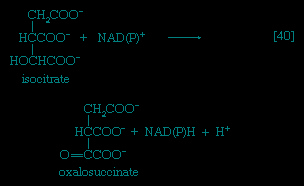 Special Comp-->
Special Comp-->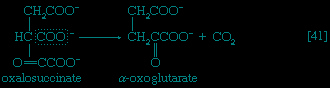 Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->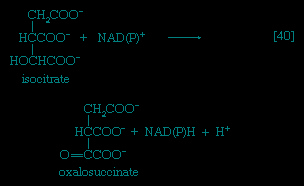 Special Comp-->
Special Comp-->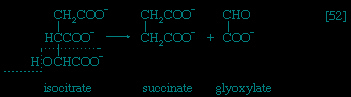 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->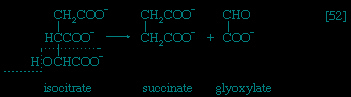 Reaction 【51-->
Reaction 【51--> 】 does not occur in facultative anaerobic organisms or in strict aerobes, however. Instead, in these organisms two molecules of acetyl coenzyme A give rise to the net synthesis of a four-carbon intermediate of the TCA cycle via a route known as the glyoxylate cycle. In this route (Figure 8-->
】 does not occur in facultative anaerobic organisms or in strict aerobes, however. Instead, in these organisms two molecules of acetyl coenzyme A give rise to the net synthesis of a four-carbon intermediate of the TCA cycle via a route known as the glyoxylate cycle. In this route (Figure 8--> ), the steps of the TCA cycle that lead to the loss of carbon dioxide (see 【40-->
), the steps of the TCA cycle that lead to the loss of carbon dioxide (see 【40-->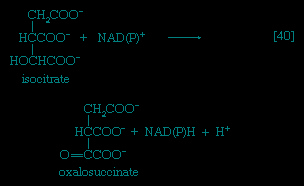 】, 【41-->
】, 【41-->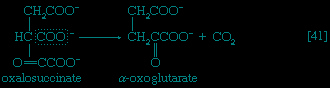 】, and 【42-->
】, and 【42--> 】) are bypassed. Instead of being oxidized to oxalosuccinate, as occurs in 【40-->
】) are bypassed. Instead of being oxidized to oxalosuccinate, as occurs in 【40-->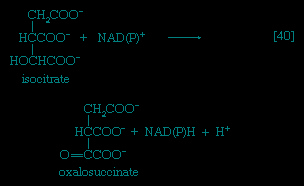 】, isocitrate is split by isocitrate lyase 【52-->
】, isocitrate is split by isocitrate lyase 【52-->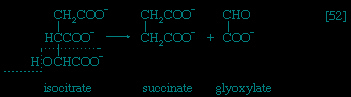 】 in a reaction similar to that of reactions 【4-->
】 in a reaction similar to that of reactions 【4--> 】 and 【15-->
】 and 【15--> 】 of carbohydrate fragmentation. The dotted line in 【52-->
】 of carbohydrate fragmentation. The dotted line in 【52-->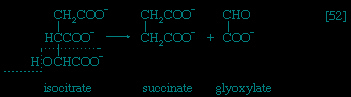 】 indicates the way in which isocitrate is split. The products are
】 indicates the way in which isocitrate is split. The products are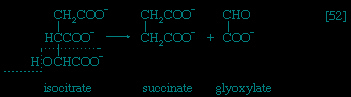
succinate and glyoxylate. Glyoxylate, like oxaloacetate, is the anion of an α-oxoacid and thus can condense, in a reaction catalyzed by malate synthase, with acetyl coenzyme A; the products of this reaction are coenzyme A and malate 【53-->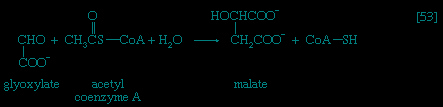 】.
】.
 】.
】.
In conjunction with the reactions of the TCA cycle that effect the re-formation of isocitrate from malate (steps 【46-->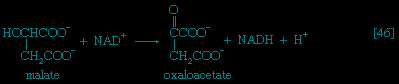 】, 【38-->
】, 【38--> 】, and 【39-->
】, and 【39-->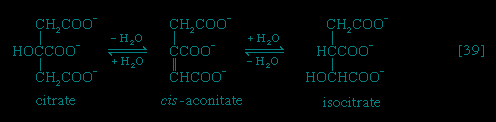 】), steps 【52-->
】), steps 【52-->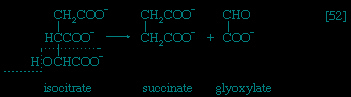 】 and 【53-->
】 and 【53--> 】 lead to the net production of a four-carbon compound (malate) from two two-carbon units (glyoxylate and acetyl coenzyme A). The sequence thus complements the TCA cycle, enabling the cycle to fulfill the dual roles of providing both energy and biosynthetic building blocks when the sole carbon source is a two-carbon compound such as acetate.
】 lead to the net production of a four-carbon compound (malate) from two two-carbon units (glyoxylate and acetyl coenzyme A). The sequence thus complements the TCA cycle, enabling the cycle to fulfill the dual roles of providing both energy and biosynthetic building blocks when the sole carbon source is a two-carbon compound such as acetate.
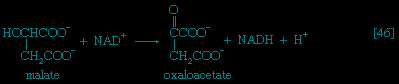 】, 【38-->
】, 【38--> 】, and 【39-->
】, and 【39--> 】), steps 【52-->
】), steps 【52-->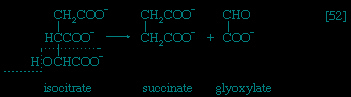 】 and 【53-->
】 and 【53--> 】 lead to the net production of a four-carbon compound (malate) from two two-carbon units (glyoxylate and acetyl coenzyme A). The sequence thus complements the TCA cycle, enabling the cycle to fulfill the dual roles of providing both energy and biosynthetic building blocks when the sole carbon source is a two-carbon compound such as acetate.
】 lead to the net production of a four-carbon compound (malate) from two two-carbon units (glyoxylate and acetyl coenzyme A). The sequence thus complements the TCA cycle, enabling the cycle to fulfill the dual roles of providing both energy and biosynthetic building blocks when the sole carbon source is a two-carbon compound such as acetate.Growth of microorganisms on TCA cycle intermediates
Most aerobic microorganisms grow readily on substances such as succinate or malate as their sole source of carbon. Under these circumstances, the formation of the intermediates of carbohydrate metabolism requires an enzymatic step ancillary to the central pathways. In most cases this step is catalyzed by phosphoenolpyruvate (PEP) carboxykinase 【54-->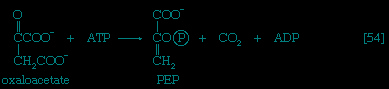 】. Oxaloacetate is decarboxylated (i.e., carbon dioxide is removed) during this energy-requiring reaction. The energy may be supplied by ATP or a similar substance (e.g., GTP) that can readily be derived from it via a reaction of the type shown in 【43a-->
】. Oxaloacetate is decarboxylated (i.e., carbon dioxide is removed) during this energy-requiring reaction. The energy may be supplied by ATP or a similar substance (e.g., GTP) that can readily be derived from it via a reaction of the type shown in 【43a--> 】. The products are PEP, carbon dioxide, and ADP.
】. The products are PEP, carbon dioxide, and ADP.
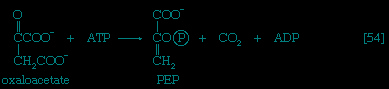 】. Oxaloacetate is decarboxylated (i.e., carbon dioxide is removed) during this energy-requiring reaction. The energy may be supplied by ATP or a similar substance (e.g., GTP) that can readily be derived from it via a reaction of the type shown in 【43a-->
】. Oxaloacetate is decarboxylated (i.e., carbon dioxide is removed) during this energy-requiring reaction. The energy may be supplied by ATP or a similar substance (e.g., GTP) that can readily be derived from it via a reaction of the type shown in 【43a--> 】. The products are PEP, carbon dioxide, and ADP.
】. The products are PEP, carbon dioxide, and ADP.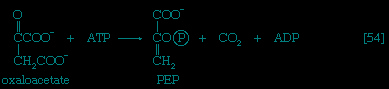
Another reaction that can yield an intermediate of carbohydrate catabolism is catalyzed by the so-called malic enzyme; in this reaction, malate is decarboxylated to pyruvate, with concomitant reduction of NADP+ 【55-->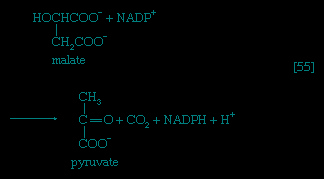 】. The primary role of malic enzyme, however, may be to generate reduced NADP+ for biosynthesis rather than to form an intermediate of carbohydrate catabolism.
】. The primary role of malic enzyme, however, may be to generate reduced NADP+ for biosynthesis rather than to form an intermediate of carbohydrate catabolism.
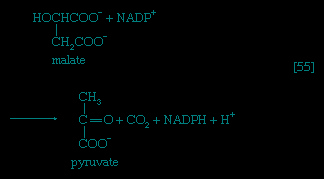 】. The primary role of malic enzyme, however, may be to generate reduced NADP+ for biosynthesis rather than to form an intermediate of carbohydrate catabolism.
】. The primary role of malic enzyme, however, may be to generate reduced NADP+ for biosynthesis rather than to form an intermediate of carbohydrate catabolism.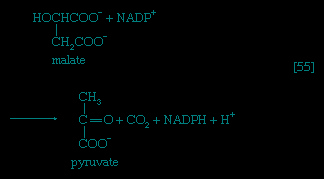
The synthesis of building blocks
gluconeogenesis
 The formation of sugars from noncarbohydrate precursors, gluconeogenesis, is of major importance in all living organisms. In the light, photosynthetic plants and microorganisms incorporate, or fix, carbon dioxide onto a five-carbon sugar and, via a sequence of transfer reactions, re-form the same sugar while also effecting the net synthesis of the glycolytic intermediate, 3-phosphoglycerate (see photosynthesis: The process of photosynthesis: carbon fixation and reduction (photosynthesis)). Phosphoglycerate is the precursor of starch, cell-wall carbohydrates, and other plant polysaccharides. A situation similar in principle applies to the growth of microorganisms on precursors of acetyl coenzyme A (Figure 8-->
The formation of sugars from noncarbohydrate precursors, gluconeogenesis, is of major importance in all living organisms. In the light, photosynthetic plants and microorganisms incorporate, or fix, carbon dioxide onto a five-carbon sugar and, via a sequence of transfer reactions, re-form the same sugar while also effecting the net synthesis of the glycolytic intermediate, 3-phosphoglycerate (see photosynthesis: The process of photosynthesis: carbon fixation and reduction (photosynthesis)). Phosphoglycerate is the precursor of starch, cell-wall carbohydrates, and other plant polysaccharides. A situation similar in principle applies to the growth of microorganisms on precursors of acetyl coenzyme A (Figure 8--> ) or on intermediates of the TCA cycle—that is, a large variety of cell components are derived from carbohydrates that, in turn, are synthesized from these noncarbohydrate precursors. Higher organisms also readily convert glucogenic amino acids (i.e., those that do not yield acetyl coenzyme A as a catabolic product) into TCA cycle intermediates, which are then converted into glucose. The amounts of glucose thus transformed depend on the needs of the organism for protein synthesis and on the availability of fuels other than glucose. The synthesis of blood glucose from lactate, which occurs largely in liver, is a particularly active process during recovery from intense muscular activity.
) or on intermediates of the TCA cycle—that is, a large variety of cell components are derived from carbohydrates that, in turn, are synthesized from these noncarbohydrate precursors. Higher organisms also readily convert glucogenic amino acids (i.e., those that do not yield acetyl coenzyme A as a catabolic product) into TCA cycle intermediates, which are then converted into glucose. The amounts of glucose thus transformed depend on the needs of the organism for protein synthesis and on the availability of fuels other than glucose. The synthesis of blood glucose from lactate, which occurs largely in liver, is a particularly active process during recovery from intense muscular activity.Special Comp--> Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->
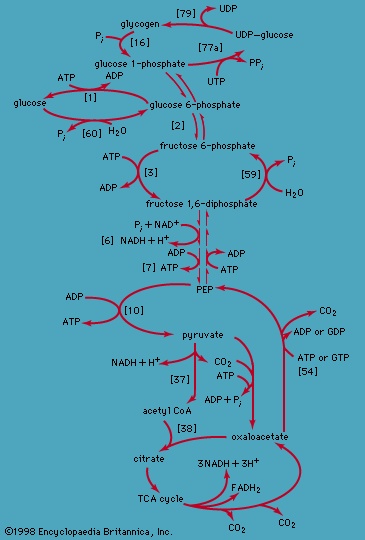 Most of the steps in the pathway for the biosynthesis of glucose from pyruvate are catalyzed by the enzymes of glycolysis; the direction of the reactions is reversed. Three virtually irreversible steps in glucose catabolism (see 【10-->
Most of the steps in the pathway for the biosynthesis of glucose from pyruvate are catalyzed by the enzymes of glycolysis; the direction of the reactions is reversed. Three virtually irreversible steps in glucose catabolism (see 【10--> 】, 【3-->
】, 【3--> 】, and 【1-->
】, and 【1--> 】) that cannot be utilized in gluconeogenesis, however, are bypassed by alternative reactions that tend to proceed in the direction of glucose synthesis (Figure 9-->
】) that cannot be utilized in gluconeogenesis, however, are bypassed by alternative reactions that tend to proceed in the direction of glucose synthesis (Figure 9--> ).
).
 Special Comp-->
Special Comp--> Special Comp-->
Special Comp-->
 Most of the steps in the pathway for the biosynthesis of glucose from pyruvate are catalyzed by the enzymes of glycolysis; the direction of the reactions is reversed. Three virtually irreversible steps in glucose catabolism (see 【10-->
Most of the steps in the pathway for the biosynthesis of glucose from pyruvate are catalyzed by the enzymes of glycolysis; the direction of the reactions is reversed. Three virtually irreversible steps in glucose catabolism (see 【10--> 】, 【3-->
】, 【3--> 】, and 【1-->
】, and 【1--> 】) that cannot be utilized in gluconeogenesis, however, are bypassed by alternative reactions that tend to proceed in the direction of glucose synthesis (Figure 9-->
】) that cannot be utilized in gluconeogenesis, however, are bypassed by alternative reactions that tend to proceed in the direction of glucose synthesis (Figure 9--> ).
).Formation of PEP from pyruvate
The first alternative reaction is the conversion of pyruvate to PEP. Three mechanisms for overcoming the energy barrier associated with the direct reversal of the pyruvate kinase reaction 【10--> 】 are known. In some bacteria, PEP is formed from pyruvate by the utilization of two of the high-energy bonds of ATP; the products include, in addition to PEP, AMP and inorganic phosphate 【56-->
】 are known. In some bacteria, PEP is formed from pyruvate by the utilization of two of the high-energy bonds of ATP; the products include, in addition to PEP, AMP and inorganic phosphate 【56-->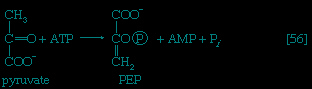 】. A variant of this reaction occurs in some bacteria, in which ATP and inorganic phosphate are reactants and AMP and inorganic pyrophosphate are products; as mentioned above, inorganic pyrophosphate is likely to be hydrolyzed to two equivalents of inorganic phosphate, so that the net balance of the reaction is identical with 【56-->
】. A variant of this reaction occurs in some bacteria, in which ATP and inorganic phosphate are reactants and AMP and inorganic pyrophosphate are products; as mentioned above, inorganic pyrophosphate is likely to be hydrolyzed to two equivalents of inorganic phosphate, so that the net balance of the reaction is identical with 【56-->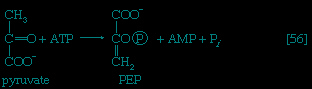 】.
】.
 】 are known. In some bacteria, PEP is formed from pyruvate by the utilization of two of the high-energy bonds of ATP; the products include, in addition to PEP, AMP and inorganic phosphate 【56-->
】 are known. In some bacteria, PEP is formed from pyruvate by the utilization of two of the high-energy bonds of ATP; the products include, in addition to PEP, AMP and inorganic phosphate 【56-->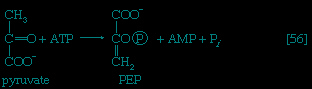 】. A variant of this reaction occurs in some bacteria, in which ATP and inorganic phosphate are reactants and AMP and inorganic pyrophosphate are products; as mentioned above, inorganic pyrophosphate is likely to be hydrolyzed to two equivalents of inorganic phosphate, so that the net balance of the reaction is identical with 【56-->
】. A variant of this reaction occurs in some bacteria, in which ATP and inorganic phosphate are reactants and AMP and inorganic pyrophosphate are products; as mentioned above, inorganic pyrophosphate is likely to be hydrolyzed to two equivalents of inorganic phosphate, so that the net balance of the reaction is identical with 【56-->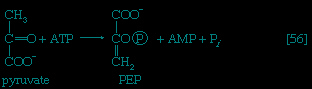 】.
】.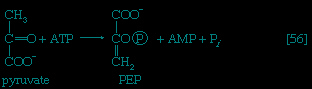
In other organisms, including many microorganisms, birds, and mammals, the formation of PEP from pyruvate is effected by the sum of reactions 【50--> 】 and 【54-->
】 and 【54-->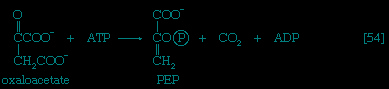 】, each of which consumes one ATP; the overall balance is shown in 【57-->
】, each of which consumes one ATP; the overall balance is shown in 【57--> 】, in which two molecules of ATP react with pyruvate to form PEP, ADP, and inorganic phosphate. The enzyme adenylate kinase catalyzes the interconversion of the various adenine nucleotides, as shown in 【58-->
】, in which two molecules of ATP react with pyruvate to form PEP, ADP, and inorganic phosphate. The enzyme adenylate kinase catalyzes the interconversion of the various adenine nucleotides, as shown in 【58--> 】.
】.
 】 and 【54-->
】 and 【54-->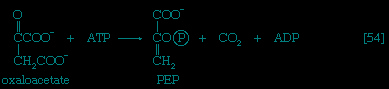 】, each of which consumes one ATP; the overall balance is shown in 【57-->
】, each of which consumes one ATP; the overall balance is shown in 【57--> 】, in which two molecules of ATP react with pyruvate to form PEP, ADP, and inorganic phosphate. The enzyme adenylate kinase catalyzes the interconversion of the various adenine nucleotides, as shown in 【58-->
】, in which two molecules of ATP react with pyruvate to form PEP, ADP, and inorganic phosphate. The enzyme adenylate kinase catalyzes the interconversion of the various adenine nucleotides, as shown in 【58--> 】.
】.
The combination of steps 【57】 and 【58】 yields the same energy balance as does the direct conversion of pyruvate to PEP 【56】.

Hydrolysis of fructose 1, 6-diphosphate and glucose 6-phosphate
The second step of glycolysis bypassed in gluconeogenesis is that catalyzed by phosphofructokinase 【3--> 】. Instead, the fructose 1,6-diphosphate synthesized from dihydroxyacetonephosphate and glyceraldehyde 3-phosphate in the reaction catalyzed by aldolase is hydrolyzed, with the loss of the phosphate group linked to the first carbon atom.
】. Instead, the fructose 1,6-diphosphate synthesized from dihydroxyacetonephosphate and glyceraldehyde 3-phosphate in the reaction catalyzed by aldolase is hydrolyzed, with the loss of the phosphate group linked to the first carbon atom.
 】. Instead, the fructose 1,6-diphosphate synthesized from dihydroxyacetonephosphate and glyceraldehyde 3-phosphate in the reaction catalyzed by aldolase is hydrolyzed, with the loss of the phosphate group linked to the first carbon atom.
】. Instead, the fructose 1,6-diphosphate synthesized from dihydroxyacetonephosphate and glyceraldehyde 3-phosphate in the reaction catalyzed by aldolase is hydrolyzed, with the loss of the phosphate group linked to the first carbon atom.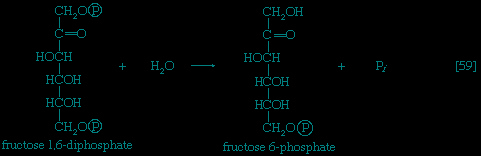
The enzyme fructose diphosphatase catalyzes the reaction 【59--> 】, in which the products are fructose 6-phosphate and inorganic phosphate. The fructose 6-phosphate thus formed is a precursor of mucopolysaccharides (polysaccharides with nitrogen-containing components). In addition, its conversion to glucose 6-phosphate provides the starting material for the formation of storage polysaccharides such as starch and glycogen, of monosaccharides other than glucose, of disaccharides (carbohydrates with two sugar components), and of some structural polysaccharides (e.g., cellulose). The maintenance of the glucose content of vertebrate blood requires glucose 6-phosphate to be converted to glucose. This process occurs in the kidney, in the lining of the intestine, and most importantly in the liver. The reaction does not occur by reversal of the hexokinase or glucokinase reactions that effect the formation of glucose 6-phosphate from glucose and ATP 【1-->
】, in which the products are fructose 6-phosphate and inorganic phosphate. The fructose 6-phosphate thus formed is a precursor of mucopolysaccharides (polysaccharides with nitrogen-containing components). In addition, its conversion to glucose 6-phosphate provides the starting material for the formation of storage polysaccharides such as starch and glycogen, of monosaccharides other than glucose, of disaccharides (carbohydrates with two sugar components), and of some structural polysaccharides (e.g., cellulose). The maintenance of the glucose content of vertebrate blood requires glucose 6-phosphate to be converted to glucose. This process occurs in the kidney, in the lining of the intestine, and most importantly in the liver. The reaction does not occur by reversal of the hexokinase or glucokinase reactions that effect the formation of glucose 6-phosphate from glucose and ATP 【1--> 】; rather, glucose 6-phosphate is hydrolyzed in a reaction catalyzed by glucose 6-phosphatase, and the phosphate is released as inorganic phosphate 【60-->
】; rather, glucose 6-phosphate is hydrolyzed in a reaction catalyzed by glucose 6-phosphatase, and the phosphate is released as inorganic phosphate 【60-->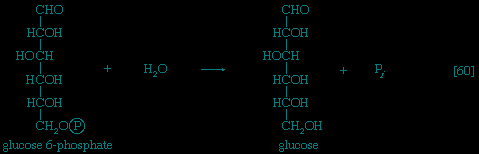 】.
】.
 】, in which the products are fructose 6-phosphate and inorganic phosphate. The fructose 6-phosphate thus formed is a precursor of mucopolysaccharides (polysaccharides with nitrogen-containing components). In addition, its conversion to glucose 6-phosphate provides the starting material for the formation of storage polysaccharides such as starch and glycogen, of monosaccharides other than glucose, of disaccharides (carbohydrates with two sugar components), and of some structural polysaccharides (e.g., cellulose). The maintenance of the glucose content of vertebrate blood requires glucose 6-phosphate to be converted to glucose. This process occurs in the kidney, in the lining of the intestine, and most importantly in the liver. The reaction does not occur by reversal of the hexokinase or glucokinase reactions that effect the formation of glucose 6-phosphate from glucose and ATP 【1-->
】, in which the products are fructose 6-phosphate and inorganic phosphate. The fructose 6-phosphate thus formed is a precursor of mucopolysaccharides (polysaccharides with nitrogen-containing components). In addition, its conversion to glucose 6-phosphate provides the starting material for the formation of storage polysaccharides such as starch and glycogen, of monosaccharides other than glucose, of disaccharides (carbohydrates with two sugar components), and of some structural polysaccharides (e.g., cellulose). The maintenance of the glucose content of vertebrate blood requires glucose 6-phosphate to be converted to glucose. This process occurs in the kidney, in the lining of the intestine, and most importantly in the liver. The reaction does not occur by reversal of the hexokinase or glucokinase reactions that effect the formation of glucose 6-phosphate from glucose and ATP 【1--> 】; rather, glucose 6-phosphate is hydrolyzed in a reaction catalyzed by glucose 6-phosphatase, and the phosphate is released as inorganic phosphate 【60-->
】; rather, glucose 6-phosphate is hydrolyzed in a reaction catalyzed by glucose 6-phosphatase, and the phosphate is released as inorganic phosphate 【60--> 】.
】.
lipid components
The component building blocks of the lipids found in storage fats, in lipoproteins (combinations of lipid and protein), and in the membranes of cells and organelles are glycerol, the fatty acids, and a number of other compounds (e.g., serine, inositol).
glycerol
Glycerol is readily derived from dihydroxyacetone phosphate, an intermediate of glycolysis (see 【4--> 】). In a reaction catalyzed by glycerol 1-phosphate dehydrogenase 【61-->
】). In a reaction catalyzed by glycerol 1-phosphate dehydrogenase 【61-->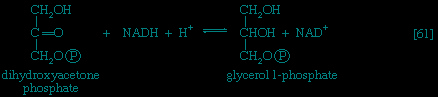 】, dihydroxyacetone phosphate is reduced to glycerol 1-phosphate; reduced NAD+ provides the reducing equivalents for the reaction and is oxidized. This compound reacts further (see below Other components (metabolism)).
】, dihydroxyacetone phosphate is reduced to glycerol 1-phosphate; reduced NAD+ provides the reducing equivalents for the reaction and is oxidized. This compound reacts further (see below Other components (metabolism)).
 】). In a reaction catalyzed by glycerol 1-phosphate dehydrogenase 【61-->
】). In a reaction catalyzed by glycerol 1-phosphate dehydrogenase 【61-->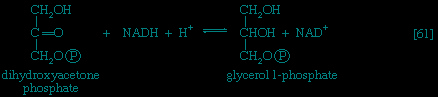 】, dihydroxyacetone phosphate is reduced to glycerol 1-phosphate; reduced NAD+ provides the reducing equivalents for the reaction and is oxidized. This compound reacts further (see below Other components (metabolism)).
】, dihydroxyacetone phosphate is reduced to glycerol 1-phosphate; reduced NAD+ provides the reducing equivalents for the reaction and is oxidized. This compound reacts further (see below Other components (metabolism)).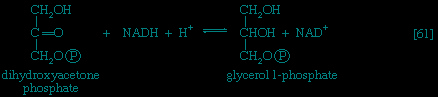
Fatty acids (fatty acid)
 Special Comp-->
Special Comp-->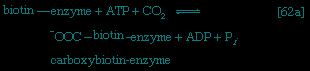 Although all the carbon atoms of the fatty acids found in lipids are derived from the acetyl coenzyme A produced by the catabolism of carbohydrates and fatty acids (Figure 2-->
Although all the carbon atoms of the fatty acids found in lipids are derived from the acetyl coenzyme A produced by the catabolism of carbohydrates and fatty acids (Figure 2--> ), the molecule first undergoes a carboxylation, forming malonyl coenzyme A, before participating in fatty acid synthesis. The carboxylation reaction is catalyzed by acetyl CoA carboxylase, an enzyme whose prosthetic group is the vitamin biotin. The biotin–enzyme first undergoes a reaction that results in the attachment of carbon dioxide to biotin; ATP is required and forms ADP and inorganic phosphate 【62a-->
), the molecule first undergoes a carboxylation, forming malonyl coenzyme A, before participating in fatty acid synthesis. The carboxylation reaction is catalyzed by acetyl CoA carboxylase, an enzyme whose prosthetic group is the vitamin biotin. The biotin–enzyme first undergoes a reaction that results in the attachment of carbon dioxide to biotin; ATP is required and forms ADP and inorganic phosphate 【62a-->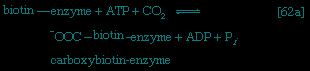 】. The complex product,
】. The complex product,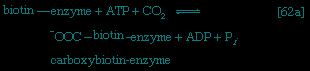
called carboxybiotin–enzyme, releases the carboxy moiety to acetyl coenzyme A, forming malonyl coenzyme A and restoring the biotin–enzyme 【62b-->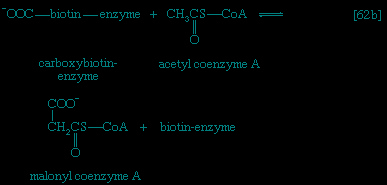 】.
】.
 】.
】.
The overall reaction 【62-->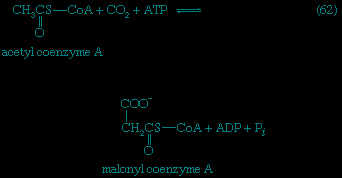 】 catalyzed by acetyl coenzyme A carboxylase thus involves the expenditure of one molecule of ATP for the formation of each molecule of malonyl coenzyme A from acetyl coenzyme A and carbon dioxide.
】 catalyzed by acetyl coenzyme A carboxylase thus involves the expenditure of one molecule of ATP for the formation of each molecule of malonyl coenzyme A from acetyl coenzyme A and carbon dioxide.
 】 catalyzed by acetyl coenzyme A carboxylase thus involves the expenditure of one molecule of ATP for the formation of each molecule of malonyl coenzyme A from acetyl coenzyme A and carbon dioxide.
】 catalyzed by acetyl coenzyme A carboxylase thus involves the expenditure of one molecule of ATP for the formation of each molecule of malonyl coenzyme A from acetyl coenzyme A and carbon dioxide.Malonyl coenzyme A and a molecule of acetyl coenzyme A react (in bacteria) with the sulfhydryl group of a relatively small molecule known as acyl-carrier protein (ACP–SH); in higher organisms ACP–SH is part of a multienzyme complex called fatty acid synthetase. ACP–SH is involved in all of the reactions leading to the synthesis of a fatty acid such as palmitic acid from acetyl coenzyme A and malonyl coenzyme A. The products of 【63a-->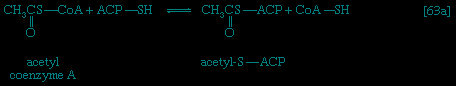 】 and 【63b-->
】 and 【63b--> 】 are acetyl-S-ACP, malonyl-S-ACP, and coenzyme A. The enzymes catalyzing 【63a-->
】 are acetyl-S-ACP, malonyl-S-ACP, and coenzyme A. The enzymes catalyzing 【63a-->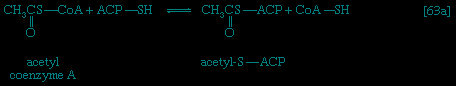 】 and 【63b-->
】 and 【63b--> 】 are known as acetyl transacylase and malonyl transacylase, respectively. Acetyl-ACP and malonyl-ACP react in a reaction catalyzed by β-ketoacyl-ACP synthetase so that the acetyl moiety (CH3CO−) is transferred to the malonyl moiety (-OOCH2CO−). Simultaneously, the carbon dioxide fixed in step 【62-->
】 are known as acetyl transacylase and malonyl transacylase, respectively. Acetyl-ACP and malonyl-ACP react in a reaction catalyzed by β-ketoacyl-ACP synthetase so that the acetyl moiety (CH3CO−) is transferred to the malonyl moiety (-OOCH2CO−). Simultaneously, the carbon dioxide fixed in step 【62--> 】 is lost, leaving as a product a four-carbon moiety attached to ACP and called acetoacetyl-S-ACP 【64-->
】 is lost, leaving as a product a four-carbon moiety attached to ACP and called acetoacetyl-S-ACP 【64-->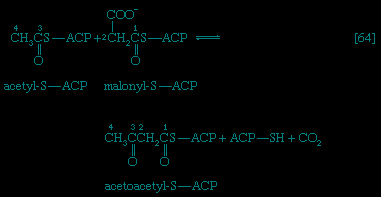 】.
】.
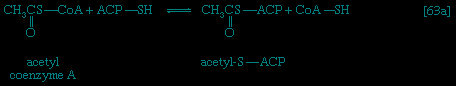 】 and 【63b-->
】 and 【63b--> 】 are acetyl-S-ACP, malonyl-S-ACP, and coenzyme A. The enzymes catalyzing 【63a-->
】 are acetyl-S-ACP, malonyl-S-ACP, and coenzyme A. The enzymes catalyzing 【63a-->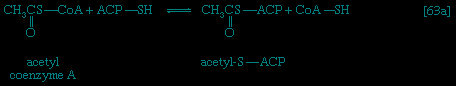 】 and 【63b-->
】 and 【63b--> 】 are known as acetyl transacylase and malonyl transacylase, respectively. Acetyl-ACP and malonyl-ACP react in a reaction catalyzed by β-ketoacyl-ACP synthetase so that the acetyl moiety (CH3CO−) is transferred to the malonyl moiety (-OOCH2CO−). Simultaneously, the carbon dioxide fixed in step 【62-->
】 are known as acetyl transacylase and malonyl transacylase, respectively. Acetyl-ACP and malonyl-ACP react in a reaction catalyzed by β-ketoacyl-ACP synthetase so that the acetyl moiety (CH3CO−) is transferred to the malonyl moiety (-OOCH2CO−). Simultaneously, the carbon dioxide fixed in step 【62--> 】 is lost, leaving as a product a four-carbon moiety attached to ACP and called acetoacetyl-S-ACP 【64-->
】 is lost, leaving as a product a four-carbon moiety attached to ACP and called acetoacetyl-S-ACP 【64--> 】.
】.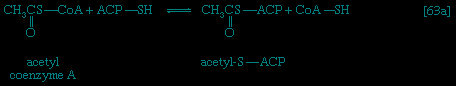

It should be noted that the carbon atoms of acetyl-S-ACP occur at the end of acetoacetyl-S-ACP (see carbon atoms numbered 4 and 3 in 【64--> 】) and that carbon dioxide plays an essentially catalytic role; the decarboxylation of the malonyl-S-ACP 【64-->
】) and that carbon dioxide plays an essentially catalytic role; the decarboxylation of the malonyl-S-ACP 【64--> 】 provides a strong thermodynamic pull toward fatty acid synthesis.
】 provides a strong thermodynamic pull toward fatty acid synthesis.
 】) and that carbon dioxide plays an essentially catalytic role; the decarboxylation of the malonyl-S-ACP 【64-->
】) and that carbon dioxide plays an essentially catalytic role; the decarboxylation of the malonyl-S-ACP 【64--> 】 provides a strong thermodynamic pull toward fatty acid synthesis.
】 provides a strong thermodynamic pull toward fatty acid synthesis.The analogy between reaction 【64--> 】 of fatty acid synthesis and the cleavage reaction 【25-->
】 of fatty acid synthesis and the cleavage reaction 【25--> 】 of fatty acid catabolism is apparent in the other reactions of fatty acid synthesis. The acetoacetyl-S-ACP, for example, undergoes reduction to β-hydroxybutyryl-S-ACP 【65-->
】 of fatty acid catabolism is apparent in the other reactions of fatty acid synthesis. The acetoacetyl-S-ACP, for example, undergoes reduction to β-hydroxybutyryl-S-ACP 【65--> 】; the reaction is catalyzed by β-ketoacyl-ACP reductase. Reduced NADP+ is the electron donor, however, and not reduced NAD+ (which would participate in the reversal of reaction 【24】). NADP+ is thus a product in 【65-->
】; the reaction is catalyzed by β-ketoacyl-ACP reductase. Reduced NADP+ is the electron donor, however, and not reduced NAD+ (which would participate in the reversal of reaction 【24】). NADP+ is thus a product in 【65--> 】. In 【66-->
】. In 【66--> 】 β-hydroxybutyryl-S-ACP is dehydrated (i.e., one molecule of water is removed), in a reaction catalyzed by enoyl-ACP-hydrase, and then undergoes a second reduction 【67-->
】 β-hydroxybutyryl-S-ACP is dehydrated (i.e., one molecule of water is removed), in a reaction catalyzed by enoyl-ACP-hydrase, and then undergoes a second reduction 【67-->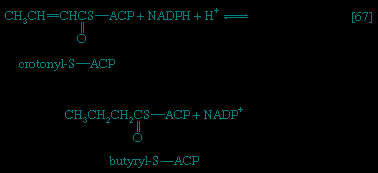 】, in which reduced NADP+
】, in which reduced NADP+
 】 of fatty acid synthesis and the cleavage reaction 【25-->
】 of fatty acid synthesis and the cleavage reaction 【25--> 】 of fatty acid catabolism is apparent in the other reactions of fatty acid synthesis. The acetoacetyl-S-ACP, for example, undergoes reduction to β-hydroxybutyryl-S-ACP 【65-->
】 of fatty acid catabolism is apparent in the other reactions of fatty acid synthesis. The acetoacetyl-S-ACP, for example, undergoes reduction to β-hydroxybutyryl-S-ACP 【65--> 】; the reaction is catalyzed by β-ketoacyl-ACP reductase. Reduced NADP+ is the electron donor, however, and not reduced NAD+ (which would participate in the reversal of reaction 【24】). NADP+ is thus a product in 【65-->
】; the reaction is catalyzed by β-ketoacyl-ACP reductase. Reduced NADP+ is the electron donor, however, and not reduced NAD+ (which would participate in the reversal of reaction 【24】). NADP+ is thus a product in 【65--> 】. In 【66-->
】. In 【66--> 】 β-hydroxybutyryl-S-ACP is dehydrated (i.e., one molecule of water is removed), in a reaction catalyzed by enoyl-ACP-hydrase, and then undergoes a second reduction 【67-->
】 β-hydroxybutyryl-S-ACP is dehydrated (i.e., one molecule of water is removed), in a reaction catalyzed by enoyl-ACP-hydrase, and then undergoes a second reduction 【67--> 】, in which reduced NADP+
】, in which reduced NADP+
again acts as the electron donor. The products of 【66--> 】 are crotonyl-S-ACP and water. The products of 【67-->
】 are crotonyl-S-ACP and water. The products of 【67--> 】, which is catalyzed by crotonyl-ACP reductase, are butyryl-S-ACP and NADP+.
】, which is catalyzed by crotonyl-ACP reductase, are butyryl-S-ACP and NADP+.
 】 are crotonyl-S-ACP and water. The products of 【67-->
】 are crotonyl-S-ACP and water. The products of 【67--> 】, which is catalyzed by crotonyl-ACP reductase, are butyryl-S-ACP and NADP+.
】, which is catalyzed by crotonyl-ACP reductase, are butyryl-S-ACP and NADP+.
The formation of butyryl-S-ACP 【67--> 】 completes the first of several cycles, in each of which one molecule of malonyl coenzyme A enters via reactions 【62-->
】 completes the first of several cycles, in each of which one molecule of malonyl coenzyme A enters via reactions 【62--> 】 and 【63b-->
】 and 【63b--> 】. In the cycle following the one ending with 【67-->
】. In the cycle following the one ending with 【67--> 】, the butyryl moiety is transferred to malonyl-S-ACP, and a molecule of carbon dioxide is again lost; a six-carbon compound results. In subsequent cycles, each of which adds two carbon atoms to the molecule via reaction 【64-->
】, the butyryl moiety is transferred to malonyl-S-ACP, and a molecule of carbon dioxide is again lost; a six-carbon compound results. In subsequent cycles, each of which adds two carbon atoms to the molecule via reaction 【64--> 】, successively longer β-oxoacyl-S-ACP derivatives are produced.
】, successively longer β-oxoacyl-S-ACP derivatives are produced.
 】 completes the first of several cycles, in each of which one molecule of malonyl coenzyme A enters via reactions 【62-->
】 completes the first of several cycles, in each of which one molecule of malonyl coenzyme A enters via reactions 【62--> 】 and 【63b-->
】 and 【63b--> 】. In the cycle following the one ending with 【67-->
】. In the cycle following the one ending with 【67--> 】, the butyryl moiety is transferred to malonyl-S-ACP, and a molecule of carbon dioxide is again lost; a six-carbon compound results. In subsequent cycles, each of which adds two carbon atoms to the molecule via reaction 【64-->
】, the butyryl moiety is transferred to malonyl-S-ACP, and a molecule of carbon dioxide is again lost; a six-carbon compound results. In subsequent cycles, each of which adds two carbon atoms to the molecule via reaction 【64--> 】, successively longer β-oxoacyl-S-ACP derivatives are produced.
】, successively longer β-oxoacyl-S-ACP derivatives are produced.
Ultimately, a molecule with 16 carbon atoms, palmityl-S-ACP, is formed. In most organisms a deacylase catalyzes the release of free palmitic acid; in a few, synthesis continues, and an acid with 18 carbon atoms is formed. The fatty acids can then react with coenzyme A (compare reaction 【21--> 】) to form fatty acyl coenzyme A, which can condense with the glycerol 1-phosphate formed in step 【61-->
】) to form fatty acyl coenzyme A, which can condense with the glycerol 1-phosphate formed in step 【61-->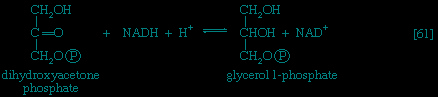 】; the product is a phosphatidic acid. The overall formation of each molecule of palmitic acid from acetyl coenzyme A—via step 【62-->
】; the product is a phosphatidic acid. The overall formation of each molecule of palmitic acid from acetyl coenzyme A—via step 【62--> 】 and repeated cycles of steps 【63】 through 【67】—requires the investment of seven molecules of ATP and 14 of reduced NADP+ (see 【68-->
】 and repeated cycles of steps 【63】 through 【67】—requires the investment of seven molecules of ATP and 14 of reduced NADP+ (see 【68-->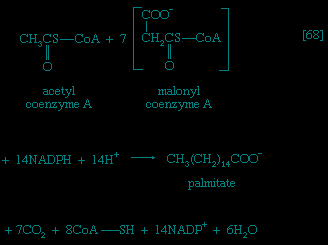 】). The process is thus an energy-requiring one (endergonic) and represents a major way by which the reducing power generated in NADP-linked dehydrogenation reactions of carbohydrate catabolism is utilized (see above The fragmentation of complex molecules: The phosphogluconate pathway (metabolism)).
】). The process is thus an energy-requiring one (endergonic) and represents a major way by which the reducing power generated in NADP-linked dehydrogenation reactions of carbohydrate catabolism is utilized (see above The fragmentation of complex molecules: The phosphogluconate pathway (metabolism)).
 】) to form fatty acyl coenzyme A, which can condense with the glycerol 1-phosphate formed in step 【61-->
】) to form fatty acyl coenzyme A, which can condense with the glycerol 1-phosphate formed in step 【61-->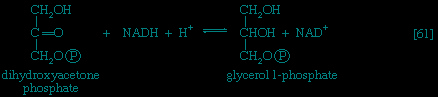 】; the product is a phosphatidic acid. The overall formation of each molecule of palmitic acid from acetyl coenzyme A—via step 【62-->
】; the product is a phosphatidic acid. The overall formation of each molecule of palmitic acid from acetyl coenzyme A—via step 【62--> 】 and repeated cycles of steps 【63】 through 【67】—requires the investment of seven molecules of ATP and 14 of reduced NADP+ (see 【68-->
】 and repeated cycles of steps 【63】 through 【67】—requires the investment of seven molecules of ATP and 14 of reduced NADP+ (see 【68--> 】). The process is thus an energy-requiring one (endergonic) and represents a major way by which the reducing power generated in NADP-linked dehydrogenation reactions of carbohydrate catabolism is utilized (see above The fragmentation of complex molecules: The phosphogluconate pathway (metabolism)).
】). The process is thus an energy-requiring one (endergonic) and represents a major way by which the reducing power generated in NADP-linked dehydrogenation reactions of carbohydrate catabolism is utilized (see above The fragmentation of complex molecules: The phosphogluconate pathway (metabolism)).
Other components
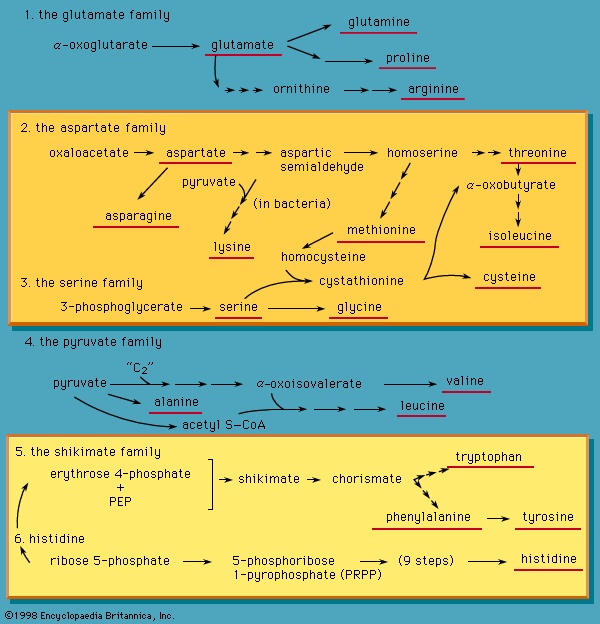 Special Comp-->
Special Comp--> The major lipids that serve as components of membranes, called phospholipids (phospholipid), as well as lipoproteins (lipoprotein), contain, in addition to two molecules of fatty acid, one molecule of a variety of different compounds. The precursors of these compounds include serine, inositol, and glycerol 1-phosphate. They are derived from intermediates of the central metabolic pathways (e.g., Figure 10-->
The major lipids that serve as components of membranes, called phospholipids (phospholipid), as well as lipoproteins (lipoprotein), contain, in addition to two molecules of fatty acid, one molecule of a variety of different compounds. The precursors of these compounds include serine, inositol, and glycerol 1-phosphate. They are derived from intermediates of the central metabolic pathways (e.g., Figure 10--> ; reaction 【61-->
; reaction 【61--> 】).
】).Amino acids
Organisms differ considerably in their ability to synthesize amino acids from the intermediates of central metabolic pathways. Most vertebrates can form only the chemically most simple amino acids; the others must be supplied in the diet. Man, for example, synthesizes about 10 of the 20 commonly encountered amino acids; these are termed nonessential amino acids. The essential amino acids must be supplied in food.
Higher plants are more versatile than animals; they can make all of the amino acids required for protein synthesis, with either ammonia (NH3) or nitrate (NO3-) as the nitrogen source. Some bacteria, and leguminous plants (e.g., peas) that harbour such bacteria in their root nodules, are able to utilize nitrogen from the air to form ammonia and use the latter for amino-acid synthesis.
Bacteria differ widely in their ability to synthesize amino acids. Some species, such as Escherichia coli, which can grow in media supplied with only a single carbon source and ammonium salts, can make all of their amino acids from these starting materials. Other bacteria may require as many as 16 different amino acids.
Each of the 20 common amino acids is synthesized by a different pathway, the complexity of which reflects the chemical complexity of the amino acid formed. As with other compounds, the pathway for the synthesis of an amino acid is for the most part different from that by which it is catabolized. A detailed discussion of the pathway by which each amino acid is formed is beyond the scope of this article, but two salient features of amino-acid biosynthesis should be mentioned.
First, ammonia is incorporated into the intermediates of metabolic pathways mainly via the glutamate dehydrogenase reaction 【28--> 】, which proceeds from right to left in biosynthetic reactions. Similarly, the transaminase enzymes (reactions 【26a-->
】, which proceeds from right to left in biosynthetic reactions. Similarly, the transaminase enzymes (reactions 【26a--> , b-->
, b--> , and c-->
, and c--> 】) enable the amino group (NH2−) to be transferred to other amino acids.
】) enable the amino group (NH2−) to be transferred to other amino acids.
 】, which proceeds from right to left in biosynthetic reactions. Similarly, the transaminase enzymes (reactions 【26a-->
】, which proceeds from right to left in biosynthetic reactions. Similarly, the transaminase enzymes (reactions 【26a--> , b-->
, b--> , and c-->
, and c--> 】) enable the amino group (NH2−) to be transferred to other amino acids.
】) enable the amino group (NH2−) to be transferred to other amino acids. Second, a group of several amino acids may be synthesized from one amino acid, which acts as a “parent” of an amino-acid “family.” The families are also interrelated in several instances. Figure 10-->
Second, a group of several amino acids may be synthesized from one amino acid, which acts as a “parent” of an amino-acid “family.” The families are also interrelated in several instances. Figure 10--> shows, for bacteria that can synthesize 20 amino acids, the way in which they are derived from intermediates of pathways already considered. Alpha-oxoglutarate and oxaloacetate are intermediates of the TCA cycle; pyruvate, 3-phosphoglycerate, and PEP are intermediates of glycolysis; and ribose 5-phosphate and erythrose 4-phosphate are formed in the phosphogluconate pathway.
shows, for bacteria that can synthesize 20 amino acids, the way in which they are derived from intermediates of pathways already considered. Alpha-oxoglutarate and oxaloacetate are intermediates of the TCA cycle; pyruvate, 3-phosphoglycerate, and PEP are intermediates of glycolysis; and ribose 5-phosphate and erythrose 4-phosphate are formed in the phosphogluconate pathway.Mononucleotides
Most organisms can synthesize the purine and pyrimidine nucleotides that serve as the building blocks of RNA (containing nucleotides in which the pentose sugar is ribose, called ribonucleotides) and DNA (containing nucleotides in which the pentose sugar is deoxyribose, called deoxyribonucleotides) as well as the agents of energy exchange.
purine ribonucleotides
The purine ribonucleotides (AMP and GMP) are derived from ribose 5-phosphate. The overall sequence that leads to the parent purine ribonucleotide, which is inosinic acid, involves 10 enzymatic steps.
 Special Comp-->
Special Comp--> Special Comp-->
Special Comp--> Figure 11-->
Figure 11--> is an outline of the manner in which inosinic acid is synthesized. Inosinic acid can be converted to AMP and GMP; these in turn yield the triphosphates (i.e., ATP and GTP) via reactions catalyzed by adenylate kinase 【69-->
is an outline of the manner in which inosinic acid is synthesized. Inosinic acid can be converted to AMP and GMP; these in turn yield the triphosphates (i.e., ATP and GTP) via reactions catalyzed by adenylate kinase 【69--> 】 and nucleoside diphosphate kinase (see reaction 【43a-->
】 and nucleoside diphosphate kinase (see reaction 【43a--> 】).
】).
pyrimidine ribonucleotides
The biosynthetic pathway for the pyrimidine nucleotides is somewhat simpler than that for the purine nucleotides. Aspartate (derived from the TCA cycle intermediate, oxaloacetate)
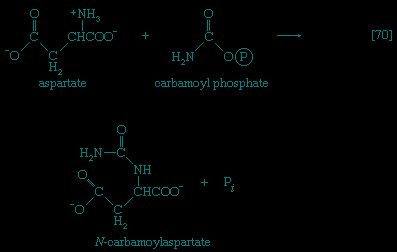
and carbamoyl phosphate (derived from carbon dioxide, ATP, and ammonia via reaction 【30-->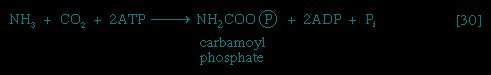 】) condense to form N-carbamoylaspartate 【70-->
】) condense to form N-carbamoylaspartate 【70--> 】, which loses water 【71-->
】, which loses water 【71-->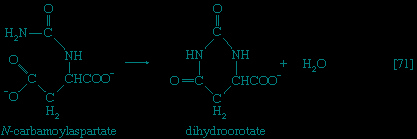 】 in a reaction catalyzed by dihydroorotase; the product, dihydroorotate,
】 in a reaction catalyzed by dihydroorotase; the product, dihydroorotate,
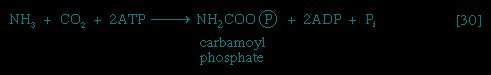 】) condense to form N-carbamoylaspartate 【70-->
】) condense to form N-carbamoylaspartate 【70--> 】, which loses water 【71-->
】, which loses water 【71--> 】 in a reaction catalyzed by dihydroorotase; the product, dihydroorotate,
】 in a reaction catalyzed by dihydroorotase; the product, dihydroorotate,
is then oxidized to orotate in a reaction catalyzed by dihydroorotic acid dehydrogenase, in which NAD+ is reduced 【72-->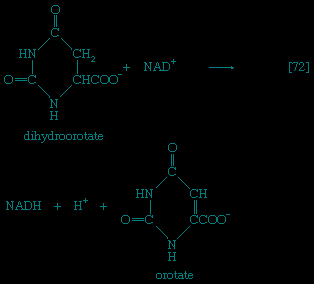 】.
】.
 】.
】.
Special Comp-->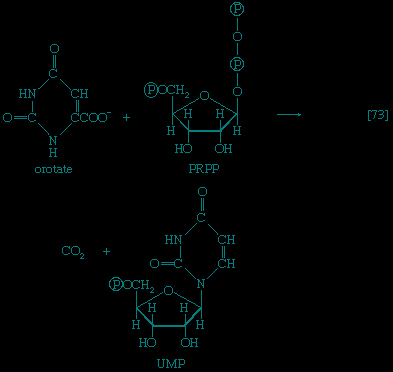

 Special Comp-->
Special Comp--> The orotate accepts a pentose phosphate moiety 【73-->
The orotate accepts a pentose phosphate moiety 【73--> 】 from 5-phosphoribose 1-pyrophosphate (PRPP); PRPP, which is formed from ribose 5-phosphate and ATP, also initiates the pathways for biosynthesis of purine nucleotides (Figure 11-->
】 from 5-phosphoribose 1-pyrophosphate (PRPP); PRPP, which is formed from ribose 5-phosphate and ATP, also initiates the pathways for biosynthesis of purine nucleotides (Figure 11--> ) and of histidine (Figure 10-->
) and of histidine (Figure 10--> ). The product loses carbon dioxide to yield the parent pyrimidine nucleotide, uridylic acid (UMP; see 【73-->
). The product loses carbon dioxide to yield the parent pyrimidine nucleotide, uridylic acid (UMP; see 【73--> 】).
】).


 Special Comp-->
Special Comp--> The orotate accepts a pentose phosphate moiety 【73-->
The orotate accepts a pentose phosphate moiety 【73--> 】 from 5-phosphoribose 1-pyrophosphate (PRPP); PRPP, which is formed from ribose 5-phosphate and ATP, also initiates the pathways for biosynthesis of purine nucleotides (Figure 11-->
】 from 5-phosphoribose 1-pyrophosphate (PRPP); PRPP, which is formed from ribose 5-phosphate and ATP, also initiates the pathways for biosynthesis of purine nucleotides (Figure 11--> ) and of histidine (Figure 10-->
) and of histidine (Figure 10--> ). The product loses carbon dioxide to yield the parent pyrimidine nucleotide, uridylic acid (UMP; see 【73-->
). The product loses carbon dioxide to yield the parent pyrimidine nucleotide, uridylic acid (UMP; see 【73--> 】).
】).Analogous to the phosphorylation of purine nucleotides (steps 【69--> 】 and 【43a-->
】 and 【43a--> 】) is the phosphorylation of UMP to UDP and thence to UTP by interaction with two molecules of ATP. Uridine triphosphate (UTP) can be converted to the other pyrimidine building block of RNA, cytidine triphosphate (CTP). In bacteria, the nitrogen for this reaction 【74-->
】) is the phosphorylation of UMP to UDP and thence to UTP by interaction with two molecules of ATP. Uridine triphosphate (UTP) can be converted to the other pyrimidine building block of RNA, cytidine triphosphate (CTP). In bacteria, the nitrogen for this reaction 【74-->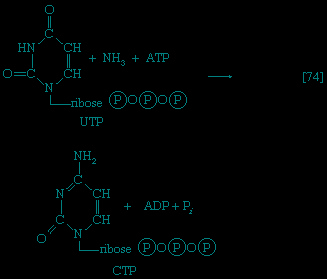 】 is derived from ammonia; in higher animals, glutamine is the nitrogen donor.
】 is derived from ammonia; in higher animals, glutamine is the nitrogen donor.
 】 and 【43a-->
】 and 【43a--> 】) is the phosphorylation of UMP to UDP and thence to UTP by interaction with two molecules of ATP. Uridine triphosphate (UTP) can be converted to the other pyrimidine building block of RNA, cytidine triphosphate (CTP). In bacteria, the nitrogen for this reaction 【74-->
】) is the phosphorylation of UMP to UDP and thence to UTP by interaction with two molecules of ATP. Uridine triphosphate (UTP) can be converted to the other pyrimidine building block of RNA, cytidine triphosphate (CTP). In bacteria, the nitrogen for this reaction 【74--> 】 is derived from ammonia; in higher animals, glutamine is the nitrogen donor.
】 is derived from ammonia; in higher animals, glutamine is the nitrogen donor.

Deoxyribonucleotides
The building blocks for the synthesis of DNA differ from those for the synthesis of RNA in two respects. In DNA the purine and pyrimidine nucleotides contain the pentose sugar 2-deoxyribose instead of ribose. In addition, the pyrimidine base uracil, found in RNA, is replaced in DNA by thymine. The deoxyribonucleoside diphosphate can be derived directly from the corresponding ribonucleoside diphosphate by a process involving the two sulfhydryl groups of the protein, thioredoxin, and a flavoprotein, thioredoxin reductase, that can in turn be reduced by reduced NADP+. Thus, for the reduction of XDP, in which X represents a purine base or cytosine, the reaction may be written as shown in 【75a-->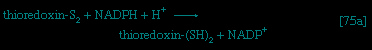 】 and 【75b-->
】 and 【75b-->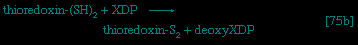 】. In 【75a-->
】. In 【75a-->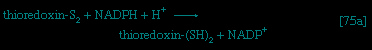 】 oxidized thioredoxin-S2 is reduced to thioredoxin-(SH)2 by NADPH, which is oxidized in the process. Thioredoxin-(SH)2 then reduces XDP to deoxyXDP in a reaction 【75b-->
】 oxidized thioredoxin-S2 is reduced to thioredoxin-(SH)2 by NADPH, which is oxidized in the process. Thioredoxin-(SH)2 then reduces XDP to deoxyXDP in a reaction 【75b-->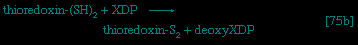 】 in which thioredoxin is re-formed.
】 in which thioredoxin is re-formed.
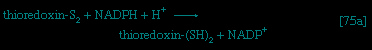 】 and 【75b-->
】 and 【75b-->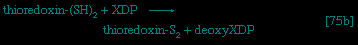 】. In 【75a-->
】. In 【75a-->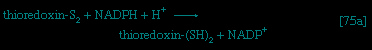 】 oxidized thioredoxin-S2 is reduced to thioredoxin-(SH)2 by NADPH, which is oxidized in the process. Thioredoxin-(SH)2 then reduces XDP to deoxyXDP in a reaction 【75b-->
】 oxidized thioredoxin-S2 is reduced to thioredoxin-(SH)2 by NADPH, which is oxidized in the process. Thioredoxin-(SH)2 then reduces XDP to deoxyXDP in a reaction 【75b-->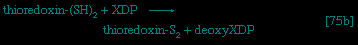 】 in which thioredoxin is re-formed.
】 in which thioredoxin is re-formed.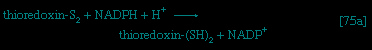
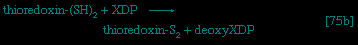
Deoxythymidylic acid (dTMP) is derived from deoxyuridylic acid (dUMP).
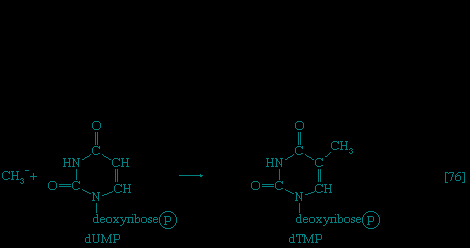
Deoxyuridine diphosphate (dUDP) is first converted to dUMP, by reaction 【69--> 】 proceeding from right to left. Deoxyuridylic acid then accepts a methyl group (CH3−) in a reaction catalyzed by an enzyme (thymidylate synthetase) with the vitamin folic acid as a coenzyme; the product is dTMP 【76-->
】 proceeding from right to left. Deoxyuridylic acid then accepts a methyl group (CH3−) in a reaction catalyzed by an enzyme (thymidylate synthetase) with the vitamin folic acid as a coenzyme; the product is dTMP 【76--> 】.
】.
 】 proceeding from right to left. Deoxyuridylic acid then accepts a methyl group (CH3−) in a reaction catalyzed by an enzyme (thymidylate synthetase) with the vitamin folic acid as a coenzyme; the product is dTMP 【76-->
】 proceeding from right to left. Deoxyuridylic acid then accepts a methyl group (CH3−) in a reaction catalyzed by an enzyme (thymidylate synthetase) with the vitamin folic acid as a coenzyme; the product is dTMP 【76--> 】.
】.The synthesis of macromolecules (macromolecule)
Carbohydrates and lipids
The formation of polysaccharides and of phospholipids from their component building blocks not only requires the investment of the energy of nucleoside triphosphates but uses these molecules in a novel manner. The biosynthetic reactions described thus far have mainly been accompanied by the formation of energy-rich intermediates (e.g., PEP in 【56-->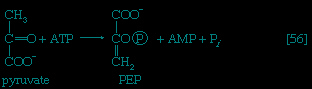 】) with the formation of either AMP or ADP; however, nucleotides serve as intermediate carriers in the formation of glycogen, starch, and a variety of lipids. This unique process necessitates reactions by which ATP, or another nucleoside triphosphate, which can be readily derived from ATP via reactions of type 【43a-->
】) with the formation of either AMP or ADP; however, nucleotides serve as intermediate carriers in the formation of glycogen, starch, and a variety of lipids. This unique process necessitates reactions by which ATP, or another nucleoside triphosphate, which can be readily derived from ATP via reactions of type 【43a--> 】, combines with a phosphorylated reactant to form a nucleoside-diphosphate product. Although the change in standard free energy is small in this reaction, the subsequent hydrolysis of the inorganic pyrophosphate also released (reaction 【21a-->
】, combines with a phosphorylated reactant to form a nucleoside-diphosphate product. Although the change in standard free energy is small in this reaction, the subsequent hydrolysis of the inorganic pyrophosphate also released (reaction 【21a-->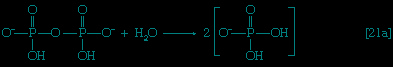 】) effectively makes the reaction irreversible in the direction of synthesis. The nucleoside triphosphate is represented as NTP in 【77】, and the phosphorylated reactant as R−Ⓟ.
】) effectively makes the reaction irreversible in the direction of synthesis. The nucleoside triphosphate is represented as NTP in 【77】, and the phosphorylated reactant as R−Ⓟ.
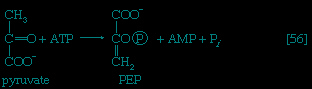 】) with the formation of either AMP or ADP; however, nucleotides serve as intermediate carriers in the formation of glycogen, starch, and a variety of lipids. This unique process necessitates reactions by which ATP, or another nucleoside triphosphate, which can be readily derived from ATP via reactions of type 【43a-->
】) with the formation of either AMP or ADP; however, nucleotides serve as intermediate carriers in the formation of glycogen, starch, and a variety of lipids. This unique process necessitates reactions by which ATP, or another nucleoside triphosphate, which can be readily derived from ATP via reactions of type 【43a--> 】, combines with a phosphorylated reactant to form a nucleoside-diphosphate product. Although the change in standard free energy is small in this reaction, the subsequent hydrolysis of the inorganic pyrophosphate also released (reaction 【21a-->
】, combines with a phosphorylated reactant to form a nucleoside-diphosphate product. Although the change in standard free energy is small in this reaction, the subsequent hydrolysis of the inorganic pyrophosphate also released (reaction 【21a-->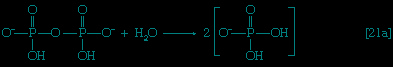 】) effectively makes the reaction irreversible in the direction of synthesis. The nucleoside triphosphate is represented as NTP in 【77】, and the phosphorylated reactant as R−Ⓟ.
】) effectively makes the reaction irreversible in the direction of synthesis. The nucleoside triphosphate is represented as NTP in 【77】, and the phosphorylated reactant as R−Ⓟ.
Reactions of type 【77】 are catalyzed by pyrophosphorylases, reaction 【21a-->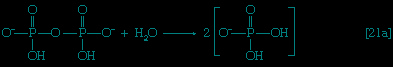 】 by inorganic pyrophosphatase.
】 by inorganic pyrophosphatase.
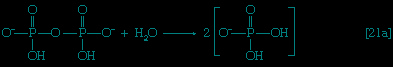 】 by inorganic pyrophosphatase.
】 by inorganic pyrophosphatase.Formation of storage polysaccharides (polysaccharide)
In the formation of storage polysaccharides—i.e., glycogen in animals, starch in plants—reaction 【77】 is preceded by the conversion of glucose 6-phosphate to glucose 1-phosphate, in a reaction catalyzed by phosph oglucomutase 【78--> 】. Glucose 1-phosphate functions as R−Ⓟ in reaction 【77a-->
】. Glucose 1-phosphate functions as R−Ⓟ in reaction 【77a-->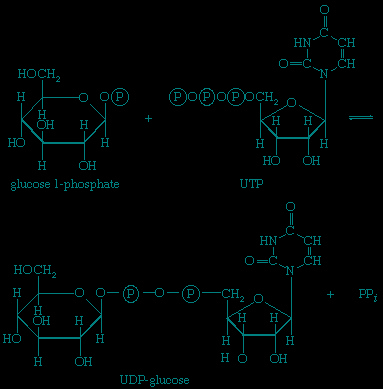 】. UTP is the specific NTP for glycogen synthesis in animals 【77a-->
】. UTP is the specific NTP for glycogen synthesis in animals 【77a--> 】;
】;
 】. Glucose 1-phosphate functions as R−Ⓟ in reaction 【77a-->
】. Glucose 1-phosphate functions as R−Ⓟ in reaction 【77a--> 】. UTP is the specific NTP for glycogen synthesis in animals 【77a-->
】. UTP is the specific NTP for glycogen synthesis in animals 【77a--> 】;
】;
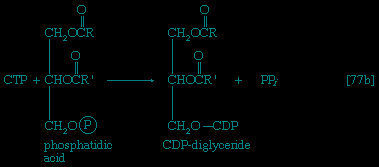

the products are UDP-glucose and pyrophosphate. In bacteria, fungi, and plants, ATP, CTP, or GTP serves instead of UTP. In all cases the nucleoside diphosphate glucose (NDP-glucose) thus synthesized can donate glucose to the terminal glucose of a polysaccharide chain, thereby increasing the number (n) of glucose molecules by one to n + 1【79】. UDP is released in this process, which is catalyzed by glycogen synthetase. starch synthesis in plants occurs by an analogous pathway catalyzed by amylose synthetase; ADP-glucose rather than UDP-glucose is the preferred glucose donor 【79a】. Similarly, cellulose, the major structural polysaccharide in plant cell walls, is synthesized in some plants by reaction 【79a】; other plants undergo analogous reactions in which GDP-glucose or CDP-glucose acts as the glucose donor.

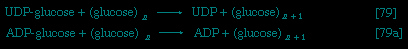
Nucleoside diphosphate sugars (sugar) also participate in the synthesis of disaccharides; for example, common table sugar, sucrose (consisting of glucose and fructose), is formed in sugarcane by the reaction sequence shown in 【80】 and 【81】;


UDP-glucose and fructose 6-phosphate first form a phosphorylated derivative of sucrose, sucrose 6′-phosphate, which is hydrolyzed to sucrose and inorganic phosphate. lactose, which consists of galactose and glucose, is the principal sugar of milk. It is synthesized in the mammary gland as shown in 【82】; UDP-galactose and glucose react to form lactose; UDP is also a product.

Formation of lipids
The neutral fats, or triglycerides (triglyceride), that constitute storage lipids, and the phospholipid components of lipoproteins and membranes, are synthesized from their building blocks by a route that branches after the first biosynthetic reaction. Initially, one molecule of glycerol 1-phosphate, the intermediate derived from carbohydrate catabolism, and two molecules of the appropriate fatty acyl coenzyme A (formed as described above, under The synthesis of building blocks: Lipid components (metabolism)) combine,

yielding phosphatidic acid 【83】. This reaction occurs preferentially with acyl coenzyme A derivatives of fatty acids containing 16 or 18 carbon atoms. In reaction 【83】, R and R′ represent the hydrocarbon moieties (Ch3(CH2)n−) of two fatty acid molecules. A triglyceride molecule (neutral fat) is formed from phosphatidic acid in a reaction catalyzed by a phosphatase that results in loss of the
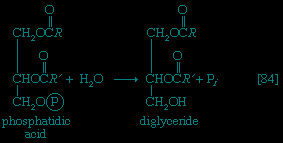
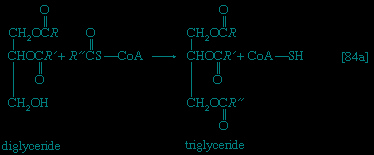
phosphate group 【84】; the diglyceride thus formed can then accept a third molecule of fatty acyl coenzyme A (represented as R″C∥OS−CoA in 【84a】).
In the biosynthesis of phospholipids, however, phosphatidic acid is not hydrolyzed; rather, it acts as the R−Ⓟ in reaction 【77--> 】, the NTP here being cytidine triphosphate (CTP). A CDP-diglyceride is produced, and inorganic pyrophosphate is released 【77b-->
】, the NTP here being cytidine triphosphate (CTP). A CDP-diglyceride is produced, and inorganic pyrophosphate is released 【77b--> 】. CDP-diglyceride is the common precursor of a variety of phospholipids. In subsequent reactions, each catalyzed by a specific enzyme, CMP is displaced from CDP-diglyceride by one of three compounds—serine, inositol, or glycerol 1-phosphate—to form CMP and, respectively, phosphatidylserine 【85a-->
】. CDP-diglyceride is the common precursor of a variety of phospholipids. In subsequent reactions, each catalyzed by a specific enzyme, CMP is displaced from CDP-diglyceride by one of three compounds—serine, inositol, or glycerol 1-phosphate—to form CMP and, respectively, phosphatidylserine 【85a-->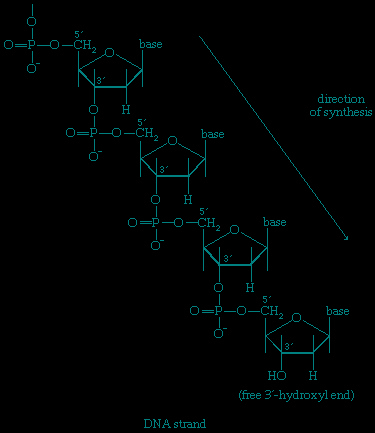 】, phosphatidylinositol 【85b-->
】, phosphatidylinositol 【85b--> 】, or, in 【85c-->
】, or, in 【85c--> 】, 3-phosphatidyl-glycerol 1′-phosphate (PGP). These reactions differ from those of polysaccharide biosynthesis (【79-->
】, 3-phosphatidyl-glycerol 1′-phosphate (PGP). These reactions differ from those of polysaccharide biosynthesis (【79--> 】, 【82-->
】, 【82--> 】) in that phosphate is retained in the phospholipid, and the nucleotide product (CMP) is therefore a nucleoside monophosphate rather than the diphosphate. These compounds can react further: phosphatidylserine to give, sequentially, phosphatidylethanolamine and phosphatidylcholine; phosphatidylinositol to yield mono- and diphosphate derivatives that are components of brain tissue and of mitochondrial membranes; and PGP to yield the phosphatidylglycerol abundant in many bacterial membranes and the diphosphatidylglycerol that is also a major component of mitochondrial and bacterial membranes.
】) in that phosphate is retained in the phospholipid, and the nucleotide product (CMP) is therefore a nucleoside monophosphate rather than the diphosphate. These compounds can react further: phosphatidylserine to give, sequentially, phosphatidylethanolamine and phosphatidylcholine; phosphatidylinositol to yield mono- and diphosphate derivatives that are components of brain tissue and of mitochondrial membranes; and PGP to yield the phosphatidylglycerol abundant in many bacterial membranes and the diphosphatidylglycerol that is also a major component of mitochondrial and bacterial membranes.
 】, the NTP here being cytidine triphosphate (CTP). A CDP-diglyceride is produced, and inorganic pyrophosphate is released 【77b-->
】, the NTP here being cytidine triphosphate (CTP). A CDP-diglyceride is produced, and inorganic pyrophosphate is released 【77b--> 】. CDP-diglyceride is the common precursor of a variety of phospholipids. In subsequent reactions, each catalyzed by a specific enzyme, CMP is displaced from CDP-diglyceride by one of three compounds—serine, inositol, or glycerol 1-phosphate—to form CMP and, respectively, phosphatidylserine 【85a-->
】. CDP-diglyceride is the common precursor of a variety of phospholipids. In subsequent reactions, each catalyzed by a specific enzyme, CMP is displaced from CDP-diglyceride by one of three compounds—serine, inositol, or glycerol 1-phosphate—to form CMP and, respectively, phosphatidylserine 【85a--> 】, phosphatidylinositol 【85b-->
】, phosphatidylinositol 【85b--> 】, or, in 【85c-->
】, or, in 【85c--> 】, 3-phosphatidyl-glycerol 1′-phosphate (PGP). These reactions differ from those of polysaccharide biosynthesis (【79-->
】, 3-phosphatidyl-glycerol 1′-phosphate (PGP). These reactions differ from those of polysaccharide biosynthesis (【79--> 】, 【82-->
】, 【82--> 】) in that phosphate is retained in the phospholipid, and the nucleotide product (CMP) is therefore a nucleoside monophosphate rather than the diphosphate. These compounds can react further: phosphatidylserine to give, sequentially, phosphatidylethanolamine and phosphatidylcholine; phosphatidylinositol to yield mono- and diphosphate derivatives that are components of brain tissue and of mitochondrial membranes; and PGP to yield the phosphatidylglycerol abundant in many bacterial membranes and the diphosphatidylglycerol that is also a major component of mitochondrial and bacterial membranes.
】) in that phosphate is retained in the phospholipid, and the nucleotide product (CMP) is therefore a nucleoside monophosphate rather than the diphosphate. These compounds can react further: phosphatidylserine to give, sequentially, phosphatidylethanolamine and phosphatidylcholine; phosphatidylinositol to yield mono- and diphosphate derivatives that are components of brain tissue and of mitochondrial membranes; and PGP to yield the phosphatidylglycerol abundant in many bacterial membranes and the diphosphatidylglycerol that is also a major component of mitochondrial and bacterial membranes.


Nucleic acids (nucleic acid) and proteins
As with the synthesis of polysaccharides and lipids, the formation of the nucleic acids and proteins from their building blocks requires the input of energy. Nucleic acids are formed from nucleoside triphosphates, with concomitant elimination of inorganic pyrophosphate, which is subsequently hydrolyzed via reaction 【21a-->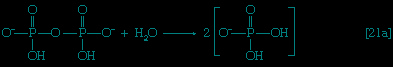 】. Amino acids also are activated, forming, at the expense of ATP, aminoacyl-complexes. This activation process is also accompanied by loss of inorganic pyrophosphate. But, although these biochemical processes are basically similar to those involved in the biosynthesis of other macromolecules, their occurrence is specifically subservient to the genetic information in DNA. DNA contains within its structure the blueprint both for its own exact duplication and for the synthesis of a number of types of RNA, among which is a class termed messenger RNA (mRNA). A complementary relationship exists between the sequence of purines (i.e., adenine and guanosine) and pyrimidines (cytosine and thymine) in the DNA comprising a gene and the sequence in mRNA into which this genetic information is transcribed. This information is then translated into the sequence of amino acids in a protein, a process that involves the functioning of a variety of other classes of ribonucleic acids (see heredity: DNA as an information carrier: transcription and translation of the genetic code (heredity)).
】. Amino acids also are activated, forming, at the expense of ATP, aminoacyl-complexes. This activation process is also accompanied by loss of inorganic pyrophosphate. But, although these biochemical processes are basically similar to those involved in the biosynthesis of other macromolecules, their occurrence is specifically subservient to the genetic information in DNA. DNA contains within its structure the blueprint both for its own exact duplication and for the synthesis of a number of types of RNA, among which is a class termed messenger RNA (mRNA). A complementary relationship exists between the sequence of purines (i.e., adenine and guanosine) and pyrimidines (cytosine and thymine) in the DNA comprising a gene and the sequence in mRNA into which this genetic information is transcribed. This information is then translated into the sequence of amino acids in a protein, a process that involves the functioning of a variety of other classes of ribonucleic acids (see heredity: DNA as an information carrier: transcription and translation of the genetic code (heredity)).
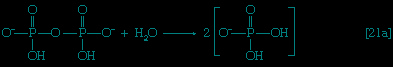 】. Amino acids also are activated, forming, at the expense of ATP, aminoacyl-complexes. This activation process is also accompanied by loss of inorganic pyrophosphate. But, although these biochemical processes are basically similar to those involved in the biosynthesis of other macromolecules, their occurrence is specifically subservient to the genetic information in DNA. DNA contains within its structure the blueprint both for its own exact duplication and for the synthesis of a number of types of RNA, among which is a class termed messenger RNA (mRNA). A complementary relationship exists between the sequence of purines (i.e., adenine and guanosine) and pyrimidines (cytosine and thymine) in the DNA comprising a gene and the sequence in mRNA into which this genetic information is transcribed. This information is then translated into the sequence of amino acids in a protein, a process that involves the functioning of a variety of other classes of ribonucleic acids (see heredity: DNA as an information carrier: transcription and translation of the genetic code (heredity)).
】. Amino acids also are activated, forming, at the expense of ATP, aminoacyl-complexes. This activation process is also accompanied by loss of inorganic pyrophosphate. But, although these biochemical processes are basically similar to those involved in the biosynthesis of other macromolecules, their occurrence is specifically subservient to the genetic information in DNA. DNA contains within its structure the blueprint both for its own exact duplication and for the synthesis of a number of types of RNA, among which is a class termed messenger RNA (mRNA). A complementary relationship exists between the sequence of purines (i.e., adenine and guanosine) and pyrimidines (cytosine and thymine) in the DNA comprising a gene and the sequence in mRNA into which this genetic information is transcribed. This information is then translated into the sequence of amino acids in a protein, a process that involves the functioning of a variety of other classes of ribonucleic acids (see heredity: DNA as an information carrier: transcription and translation of the genetic code (heredity)).Synthesis of DNA
The maintenance of genetic integrity demands not only that enzymes exist for the synthesis of DNA but that they function so as to ensure the replication of the genetic information (encoded in the DNA to be copied) with absolute fidelity. This implies that the assembly of new regions of a DNA molecule must occur on a template of DNA already present in the cell. The synthetic processes must also be capable of repairing limited regions of DNA, which may have been damaged, for example, as a consequence of exposure to ultraviolet irradiation. The physical structure of DNA is ideally adapted to its biological roles. Two strands of nucleotides are wound around each other in the form of a double helix. The helix is stabilized by hydrogen bonds that occur between the purine and pyrimidine bases of the strands. Thus, the adenine of one strand pairs with the thymine of the other, and the guanine of one strand with the cytosine of the other. The base pairs may be visualized as the treads of a spiral staircase, in which the two chains of repeating units (i.e., ribose-phosphate-ribose) form the sides.

During the biosynthesis of DNA, the two strands unwind, and each serves as a template for the synthesis of a new, complementary strand, in which the bases pair in exactly the same manner as occurred in the parent double helix. The process is catalyzed by a DNA polymerase enzyme, which catalyzes the addition of the appropriate deoxyribonucleoside triphosphate (NTP) in 【86--> 】 onto one end, specifically, the free 3′-hydroxyl end (−OH) of the growing DNA chain (see diagram of DNA strand). In 【86-->
】 onto one end, specifically, the free 3′-hydroxyl end (−OH) of the growing DNA chain (see diagram of DNA strand). In 【86--> 】 the addition of a deoxyribonucleoside monophosphate (dNMP) moiety onto a growing DNA chain (5′-DNA-polymer-3′-ΟΗ) is shown; the other product is inorganic pyrophosphate. The specific nucleotide inserted in the growing chain is dictated by the base in the complementary (template) strand of DNA with which it pairs. The functioning of DΝΑ polymerase thus requires the presence of all four deoxyribonucleoside triphosphates (i.e., dATP, dTPP, dGTP, and dCTP) as well as preformed DNA to act as a template. Although a number of DNA polymerase enzymes have been purified from different organisms, it is not yet certain whether those that have been most extensively studied are necessarily involved in the formation of new DNA molecules, or whether they are primarily concerned with the repair of damaged regions of molecules. A polynucleotide ligase that effects the formation of the phosphate bond between adjacent sugar molecules is concerned with the repair function but may also have a role in synthesis.
】 the addition of a deoxyribonucleoside monophosphate (dNMP) moiety onto a growing DNA chain (5′-DNA-polymer-3′-ΟΗ) is shown; the other product is inorganic pyrophosphate. The specific nucleotide inserted in the growing chain is dictated by the base in the complementary (template) strand of DNA with which it pairs. The functioning of DΝΑ polymerase thus requires the presence of all four deoxyribonucleoside triphosphates (i.e., dATP, dTPP, dGTP, and dCTP) as well as preformed DNA to act as a template. Although a number of DNA polymerase enzymes have been purified from different organisms, it is not yet certain whether those that have been most extensively studied are necessarily involved in the formation of new DNA molecules, or whether they are primarily concerned with the repair of damaged regions of molecules. A polynucleotide ligase that effects the formation of the phosphate bond between adjacent sugar molecules is concerned with the repair function but may also have a role in synthesis.
 】 onto one end, specifically, the free 3′-hydroxyl end (−OH) of the growing DNA chain (see diagram of DNA strand). In 【86-->
】 onto one end, specifically, the free 3′-hydroxyl end (−OH) of the growing DNA chain (see diagram of DNA strand). In 【86-->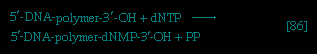 】 the addition of a deoxyribonucleoside monophosphate (dNMP) moiety onto a growing DNA chain (5′-DNA-polymer-3′-ΟΗ) is shown; the other product is inorganic pyrophosphate. The specific nucleotide inserted in the growing chain is dictated by the base in the complementary (template) strand of DNA with which it pairs. The functioning of DΝΑ polymerase thus requires the presence of all four deoxyribonucleoside triphosphates (i.e., dATP, dTPP, dGTP, and dCTP) as well as preformed DNA to act as a template. Although a number of DNA polymerase enzymes have been purified from different organisms, it is not yet certain whether those that have been most extensively studied are necessarily involved in the formation of new DNA molecules, or whether they are primarily concerned with the repair of damaged regions of molecules. A polynucleotide ligase that effects the formation of the phosphate bond between adjacent sugar molecules is concerned with the repair function but may also have a role in synthesis.
】 the addition of a deoxyribonucleoside monophosphate (dNMP) moiety onto a growing DNA chain (5′-DNA-polymer-3′-ΟΗ) is shown; the other product is inorganic pyrophosphate. The specific nucleotide inserted in the growing chain is dictated by the base in the complementary (template) strand of DNA with which it pairs. The functioning of DΝΑ polymerase thus requires the presence of all four deoxyribonucleoside triphosphates (i.e., dATP, dTPP, dGTP, and dCTP) as well as preformed DNA to act as a template. Although a number of DNA polymerase enzymes have been purified from different organisms, it is not yet certain whether those that have been most extensively studied are necessarily involved in the formation of new DNA molecules, or whether they are primarily concerned with the repair of damaged regions of molecules. A polynucleotide ligase that effects the formation of the phosphate bond between adjacent sugar molecules is concerned with the repair function but may also have a role in synthesis.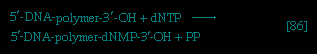
Synthesis of RNA
Various types of RNA are found in living organisms: messenger RNA (mRNA) is involved in the immediate transcription of regions of DNA; transfer RNA (tRNA) is concerned with the incorporation of amino acids into proteins; and structural RNA is found in the ribosomes that form the protein-synthesizing machinery of the cell. In cells of organisms with well-defined nuclei (i.e., eukaryotes), a heterogenous RNA fraction of unknown function is constantly broken down and resynthesized in the nucleus of the cell but does not leave it. The different types of RNA are synthesized via RNA polymerases 【87-->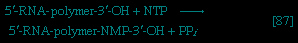 】, the action of which is analogous to that of the DNA polymerases that catalyze reaction 【86-->
】, the action of which is analogous to that of the DNA polymerases that catalyze reaction 【86-->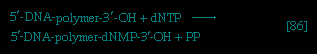 】. In 【87-->
】. In 【87-->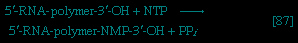 】 the growing RNA chain is represented by 5′-RNA-polymer-3′-ΟΗ, and the ribonucleoside triphosphate by NTP. One product (5′-RNA-polymer-NMP-3′-OH) reflects the incorporation of ribonucleoside monophosphate; the other product is, as in 【86-->
】 the growing RNA chain is represented by 5′-RNA-polymer-3′-ΟΗ, and the ribonucleoside triphosphate by NTP. One product (5′-RNA-polymer-NMP-3′-OH) reflects the incorporation of ribonucleoside monophosphate; the other product is, as in 【86-->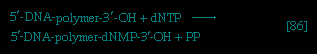 】, inorganic pyrophosphate. Synthesis of RNA requires DNA as a template, thus ensuring that the base composition of the RNA faithfully reflects that of the DNA; in addition, as in DNA synthesis, all four nucleoside triphosphates must be present. The major differences between reactions 【86-->
】, inorganic pyrophosphate. Synthesis of RNA requires DNA as a template, thus ensuring that the base composition of the RNA faithfully reflects that of the DNA; in addition, as in DNA synthesis, all four nucleoside triphosphates must be present. The major differences between reactions 【86-->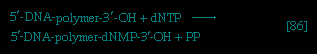 】 and 【87-->
】 and 【87-->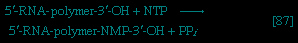 】 are that, in the latter, the nucleotides contain ribose instead of deoxyribose, and that, in RNA, uracil replaces the thymine of DNA.
】 are that, in the latter, the nucleotides contain ribose instead of deoxyribose, and that, in RNA, uracil replaces the thymine of DNA.
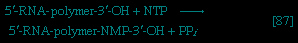 】, the action of which is analogous to that of the DNA polymerases that catalyze reaction 【86-->
】, the action of which is analogous to that of the DNA polymerases that catalyze reaction 【86-->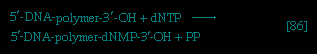 】. In 【87-->
】. In 【87-->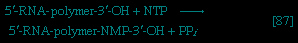 】 the growing RNA chain is represented by 5′-RNA-polymer-3′-ΟΗ, and the ribonucleoside triphosphate by NTP. One product (5′-RNA-polymer-NMP-3′-OH) reflects the incorporation of ribonucleoside monophosphate; the other product is, as in 【86-->
】 the growing RNA chain is represented by 5′-RNA-polymer-3′-ΟΗ, and the ribonucleoside triphosphate by NTP. One product (5′-RNA-polymer-NMP-3′-OH) reflects the incorporation of ribonucleoside monophosphate; the other product is, as in 【86-->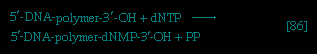 】, inorganic pyrophosphate. Synthesis of RNA requires DNA as a template, thus ensuring that the base composition of the RNA faithfully reflects that of the DNA; in addition, as in DNA synthesis, all four nucleoside triphosphates must be present. The major differences between reactions 【86-->
】, inorganic pyrophosphate. Synthesis of RNA requires DNA as a template, thus ensuring that the base composition of the RNA faithfully reflects that of the DNA; in addition, as in DNA synthesis, all four nucleoside triphosphates must be present. The major differences between reactions 【86-->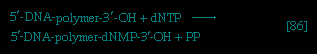 】 and 【87-->
】 and 【87-->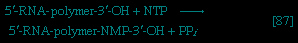 】 are that, in the latter, the nucleotides contain ribose instead of deoxyribose, and that, in RNA, uracil replaces the thymine of DNA.
】 are that, in the latter, the nucleotides contain ribose instead of deoxyribose, and that, in RNA, uracil replaces the thymine of DNA.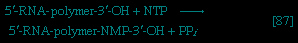
It appears that, although only one strand of the DNA double helix serves as template during the formation of RNA, some regions are transcribed from one strand, some from the other.
An important constraint on RNA synthesis is that the accurate copying of the appropriate DNA strand by RNA polymerase must start at the beginning of a gene—and not somewhere along it—and must stop as soon as the genetic information has been transcribed. The way in which this selectivity is achieved is not yet fully understood, although it has been established that E. coli contains a protein, the sigma factor, that is not required for the incorporation of the nucleoside triphosphates into the growing RNA chain but apparently is essential for binding RNA polymerase to the proper DNA sites to initiate RNA synthesis. After the initiation step, the sigma factor is released; the role of the sigma factor in transcription suggests that the DNA at the initiation sites must be unique in some way so as to ensure that the correct strand is used as the template. Evidence indicates further that other protein factors are involved in the termination of transcription.
Synthesis of proteins
Approximately 120 macromolecules are involved directly or indirectly in the process of the translation of the base sequence of a messenger RNA molecule into the amino-acid sequence of a protein. The relationship between the base sequence and the amino-acid sequence constitutes the genetic code. The basic properties of the code are: it is triplet—i.e., a linear sequence of three bases in mRNA specifies one amino acid in a protein; it is nonoverlapping—i.e., each triplet is discrete and does not overlap either neighbour; it is degenerate—i.e., many of the 20 amino acids are specified by more than one of the 64 possible triplets of bases; and it appears to apply universally to all living organisms.
The main sequence of events associated with the expression of this genetic code, as elucidated for E. coli, may be summarized as follows (see also heredity: The physical basis of heredity: Molecular genetics (heredity)).
1. Messenger RNA binds to the smaller of two subunits of large particles termed ribosomes (ribosome).
2. The amino acid that begins the assembly of the protein chain is activated and transferred to a specific transfer RNA (tRNA). The activation step, catalyzed by an aminoacyl–tRNA synthetase specific for a particular amino acid, effects the formation of an aminoacyl–AMP complex 【88a--> 】 in a manner somewhat analogous to reaction 【77-->
】 in a manner somewhat analogous to reaction 【77--> 】; ATP is required, and inorganic pyrophosphate is a product. The aminoacyl–AMP, which remains bound to the enzyme, is transferred to a specific molecule of tRNA in a reaction catalyzed by the same enzyme. AMP is released, and the other product is called aminoacyl–tRNA 【88b-->
】; ATP is required, and inorganic pyrophosphate is a product. The aminoacyl–AMP, which remains bound to the enzyme, is transferred to a specific molecule of tRNA in a reaction catalyzed by the same enzyme. AMP is released, and the other product is called aminoacyl–tRNA 【88b-->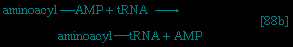 】. In E. coli the amino acid that begins the assembly of the protein is always formylmethionine (f-Met). There is no evidence that f-Met is involved in protein synthesis in eukaryotic cells.
】. In E. coli the amino acid that begins the assembly of the protein is always formylmethionine (f-Met). There is no evidence that f-Met is involved in protein synthesis in eukaryotic cells.
 】 in a manner somewhat analogous to reaction 【77-->
】 in a manner somewhat analogous to reaction 【77--> 】; ATP is required, and inorganic pyrophosphate is a product. The aminoacyl–AMP, which remains bound to the enzyme, is transferred to a specific molecule of tRNA in a reaction catalyzed by the same enzyme. AMP is released, and the other product is called aminoacyl–tRNA 【88b-->
】; ATP is required, and inorganic pyrophosphate is a product. The aminoacyl–AMP, which remains bound to the enzyme, is transferred to a specific molecule of tRNA in a reaction catalyzed by the same enzyme. AMP is released, and the other product is called aminoacyl–tRNA 【88b-->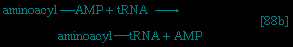 】. In E. coli the amino acid that begins the assembly of the protein is always formylmethionine (f-Met). There is no evidence that f-Met is involved in protein synthesis in eukaryotic cells.
】. In E. coli the amino acid that begins the assembly of the protein is always formylmethionine (f-Met). There is no evidence that f-Met is involved in protein synthesis in eukaryotic cells.
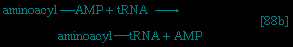
3. Aminoacyl–tRNA binds to the mRNA-ribosomal complex in a reaction in which energy is provided by the hydrolysis of GTP to GDP and inorganic phosphate. In this step and in 5 below, the genetic code is translated. All of the different tRNAs contain triplets of bases that pair specifically with the complementary base triplets in mRNA; the base triplets in mRNA specify the amino acids to be added to the protein chain. During or shortly after the pairing occurs the aminoacyl–tRNA moves from the aminoacyl-acceptor (A) site on the ribosome to another site, called a peptidyl-donor (P) site.
4. The larger subunit of the ribosome then joins the mRNA–f-Met–tRNA–smaller ribosomal subunit complex.
5. The second amino acid to be added to the protein chain is specified by the triplet of bases adjacent to the initiator triplet in mRNA. The amino acid is activated and transferred to its tRNA by a repetition of reactions 【88a--> 】 and 【88b-->
】 and 【88b-->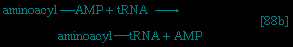 】. This newly formed aminoacyl–tRNA now binds to the A site of the mRNA–ribosome complex, with concomitant hydrolysis of GTP.
】. This newly formed aminoacyl–tRNA now binds to the A site of the mRNA–ribosome complex, with concomitant hydrolysis of GTP.
 】 and 【88b-->
】 and 【88b-->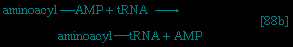 】. This newly formed aminoacyl–tRNA now binds to the A site of the mRNA–ribosome complex, with concomitant hydrolysis of GTP.
】. This newly formed aminoacyl–tRNA now binds to the A site of the mRNA–ribosome complex, with concomitant hydrolysis of GTP.6. The enzyme peptidyl transferase, which is part of the larger of the two ribosomal subunits, catalyzes the transfer of formylmethionine from the tRNA to which it is attached (designated tRNAf-Met) to the second amino acid; for example, if the second amino acid were leucine, step 5 would have achieved the binding of leucyl–tRNA (Leu–tRNALeu) next to f-Met–tRNAf-Met on the ribosome–mRNA complex. Step 6 catalyzes the transfer reaction that is shown in 【89-->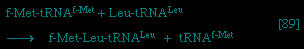 】, in which tRNAf-Met is released from formyl-methionine (f-Met), and Leu–tRNALeu is bound to formyl-methionine.
】, in which tRNAf-Met is released from formyl-methionine (f-Met), and Leu–tRNALeu is bound to formyl-methionine.
 】, in which tRNAf-Met is released from formyl-methionine (f-Met), and Leu–tRNALeu is bound to formyl-methionine.
】, in which tRNAf-Met is released from formyl-methionine (f-Met), and Leu–tRNALeu is bound to formyl-methionine.
7. In the next step three results are achieved. The dipeptide f-Met–Leu (a dipeptide consists of two amino acids) moves from the A (aminoacyl-acceptor) site to the P (peptidyl-donor) site on the ribosome; the tRNAf-Met is thereby displaced from the P site, and the ribosome moves the length of one triplet (three bases) along the mRNA molecule. The occurrence of these events is accompanied by the hydrolysis of a second molecule of GTP and leaves the system ready to receive the next aminoacyl–tRNA (by repetition of step 5). The cycle of events in 5, 6, and 7 is repeated until the ribosome moves to a triplet on the mRNA that does not specify an amino acid but provides the signal for termination of the amino-acid chain. Triplets of this type are represented by one uracil (U) preceding, and adjacent to, two adenines (UAA) or preceding one adenine and one guanosine in either order (UGA, or UAG).
8. At the termination of synthesis the completed protein is released from the tRNA to which it had remained linked. Two further events then occur in E. coli. First, the formyl constituent of the f-methionyl moiety is hydrolyzed by the catalytic action of a formylase, producing a protein with methionine at the end. If the required protein does not contain methionine in this position (and the majority of proteins in E. coli appear to), the methionine and possibly other amino acids that follow it are removed by enzymatic reactions. Second, the ribosome–mRNA complex dissociates, and the ribosomal subunits become available for a new round of translation by binding another mRNA molecule, step 1.
For the sake of brevity, other ancillary protein factors that participate in this sequence 1 to 8 have been omitted; the role of many of these factors is as yet poorly understood.
Regulation of metabolism
Fine control
The flux of nutrients along each metabolic pathway is governed chiefly by two factors: (1) the availability of substrates on which pacemaker, or key, enzymes of the pathway can act and (2) the intracellular levels of specific metabolites that affect the reaction rates of pacemaker enzymes. Key enzymes are usually complex proteins that, in addition to the site at which the catalytic process occurs (i.e., the active site), contain sites to which the regulatory metabolites bind. Interactions with the appropriate molecules at these regulatory sites cause changes in the shape of the enzyme molecule. Such changes may either facilitate or hinder the changes that occur at the active site. The rate of the enzymatic reaction is thus speeded up or slowed down by the presence of a regulatory metabolite.
In many cases, the specific small molecules that bind to the regulatory sites have no obvious structural similarity to the substrates of the enzymes; these small molecules are therefore termed allosteric (allosteric control) effectors, and the regulatory sites are termed allosteric sites. Allosteric effectors may be formed by enzyme-catalyzed reactions in the same pathway in which the enzyme regulated by the effectors functions. In this case a rise in the level of the allosteric effector would affect the flux of nutrients along that pathway in a manner analogous to the feedback phenomena of homeostatic (homeostasis) processes. Such effectors may also be formed by enzymatic reactions in apparently unrelated pathways. In this instance the rate at which one metabolic pathway operates would be profoundly affected by the rate of nutrient flux along another. It is this situation that, to a large extent, governs the sensitive and immediately responsive coordination of the many metabolic routes in the cell.
End-product inhibition

 A biosynthetic pathway is usually controlled by an allosteric effector produced as the end product of that pathway, and the pacemaker enzyme on which the effector acts usually catalyzes the first step that uniquely leads to the end product. This phenomenon, called end-product inhibition, is illustrated by the multienzyme, branched pathway for the formation from oxaloacetate of the “aspartate family” of amino acids (Figure 10-->
A biosynthetic pathway is usually controlled by an allosteric effector produced as the end product of that pathway, and the pacemaker enzyme on which the effector acts usually catalyzes the first step that uniquely leads to the end product. This phenomenon, called end-product inhibition, is illustrated by the multienzyme, branched pathway for the formation from oxaloacetate of the “aspartate family” of amino acids (Figure 10--> ). The system of interlocking controls is described in greater detail in Figure 12-->
). The system of interlocking controls is described in greater detail in Figure 12--> . As mentioned previously in this article, only plants and microorganisms can synthesize many of these amino acids, most animals requiring such amino acids to be supplied preformed in their diets.
. As mentioned previously in this article, only plants and microorganisms can synthesize many of these amino acids, most animals requiring such amino acids to be supplied preformed in their diets.
 Figure 12-->
Figure 12--> shows that there are a number of pacemaker enzymes in the biosynthetic route for the aspartate family of amino acids, most of which are uniquely involved in the formation of one product. Each of the enzymes functions after a branch point in the pathway, and all are inhibited specifically by the end product that emerges from the branch point. It is not difficult to visualize from Figure 12-->
shows that there are a number of pacemaker enzymes in the biosynthetic route for the aspartate family of amino acids, most of which are uniquely involved in the formation of one product. Each of the enzymes functions after a branch point in the pathway, and all are inhibited specifically by the end product that emerges from the branch point. It is not difficult to visualize from Figure 12--> how the supplies of lysine, methionine, and isoleucine required by a cell can be independently regulated. threonine, however, is both an amino acid essential for protein synthesis and a precursor of isoleucine. If the rate of synthesis of threonine from aspartate were regulated as are the rates of lysine, methionine, and isoleucine, an imbalance in the supply of isoleucine might result. This risk is overcome in E. coli by the existence of three different aspartokinase enzymes, all of which catalyze the first step common to the production of all the products derived from aspartate. Each has a different regulatory effector molecule. Thus, one type of aspartokinase is inhibited by lysine, a second by threonine. The third kinase is not inhibited by any naturally occurring amino acid, but its rate of synthesis (see below) is controlled by the concentration of methionine within the cell. The triple control mechanism resulting from the three different aspartokinases ensures that the accumulation of one amino acid does not shut off the supply of aspartyl phosphate necessary for the synthesis of the others.
how the supplies of lysine, methionine, and isoleucine required by a cell can be independently regulated. threonine, however, is both an amino acid essential for protein synthesis and a precursor of isoleucine. If the rate of synthesis of threonine from aspartate were regulated as are the rates of lysine, methionine, and isoleucine, an imbalance in the supply of isoleucine might result. This risk is overcome in E. coli by the existence of three different aspartokinase enzymes, all of which catalyze the first step common to the production of all the products derived from aspartate. Each has a different regulatory effector molecule. Thus, one type of aspartokinase is inhibited by lysine, a second by threonine. The third kinase is not inhibited by any naturally occurring amino acid, but its rate of synthesis (see below) is controlled by the concentration of methionine within the cell. The triple control mechanism resulting from the three different aspartokinases ensures that the accumulation of one amino acid does not shut off the supply of aspartyl phosphate necessary for the synthesis of the others.Another example of control through end-product inhibition also illustrates the manner in which the operation of two biosynthetic pathways may be coordinated. Both DNA and the various types of RNA are assembled from purine and pyrimidine nucleotides (see above The synthesis of macromolecules: Nucleic acids and proteins (metabolism)); these in turn are built up from intermediates of central metabolic pathways (see above The synthesis of building blocks: Mononucleotides (metabolism)). The first step in the synthesis of pyrimidine nucleotides is that catalyzed by aspartate carbamoyltransferase 【70a】. This step initiates a sequence of reactions that leads to the formation of pyrimidine nucleotides such as UTP and CTP 【74--> 】. Studies of aspartate carbamoyltransferase have revealed that the affinity of this enzyme for its substrate (aspartate) is markedly decreased by the presence of CTP. This effect can be overcome by the addition of ATP, a purine nucleotide. The enzyme can be dissociated into two subunits: one contains the enzymatic activity and (in the dissociated form) does not bind CTP; the other binds CTP but has no catalytic activity. Apart from providing physical evidence that pacemaker enzymes contain distinct catalytic and regulatory sites, the interaction of aspartate carbamoyltransferase with the different nucleotides provides an explanation for the control of the supply of nucleic acid precursors. If a cell contains sufficient pyrimidine nucleotides (e.g., UTP), aspartate carbamoyltransferase, the first enzyme of pyrimidine biosynthesis, is inhibited. If, however, the cell contains high levels of purine nucleotides (e.g., ATP), as required for the formation of nucleic acids, the inhibition of aspartate carbamoyltransferase is relieved, and pyrimidines are formed.
】. Studies of aspartate carbamoyltransferase have revealed that the affinity of this enzyme for its substrate (aspartate) is markedly decreased by the presence of CTP. This effect can be overcome by the addition of ATP, a purine nucleotide. The enzyme can be dissociated into two subunits: one contains the enzymatic activity and (in the dissociated form) does not bind CTP; the other binds CTP but has no catalytic activity. Apart from providing physical evidence that pacemaker enzymes contain distinct catalytic and regulatory sites, the interaction of aspartate carbamoyltransferase with the different nucleotides provides an explanation for the control of the supply of nucleic acid precursors. If a cell contains sufficient pyrimidine nucleotides (e.g., UTP), aspartate carbamoyltransferase, the first enzyme of pyrimidine biosynthesis, is inhibited. If, however, the cell contains high levels of purine nucleotides (e.g., ATP), as required for the formation of nucleic acids, the inhibition of aspartate carbamoyltransferase is relieved, and pyrimidines are formed.
 】. Studies of aspartate carbamoyltransferase have revealed that the affinity of this enzyme for its substrate (aspartate) is markedly decreased by the presence of CTP. This effect can be overcome by the addition of ATP, a purine nucleotide. The enzyme can be dissociated into two subunits: one contains the enzymatic activity and (in the dissociated form) does not bind CTP; the other binds CTP but has no catalytic activity. Apart from providing physical evidence that pacemaker enzymes contain distinct catalytic and regulatory sites, the interaction of aspartate carbamoyltransferase with the different nucleotides provides an explanation for the control of the supply of nucleic acid precursors. If a cell contains sufficient pyrimidine nucleotides (e.g., UTP), aspartate carbamoyltransferase, the first enzyme of pyrimidine biosynthesis, is inhibited. If, however, the cell contains high levels of purine nucleotides (e.g., ATP), as required for the formation of nucleic acids, the inhibition of aspartate carbamoyltransferase is relieved, and pyrimidines are formed.
】. Studies of aspartate carbamoyltransferase have revealed that the affinity of this enzyme for its substrate (aspartate) is markedly decreased by the presence of CTP. This effect can be overcome by the addition of ATP, a purine nucleotide. The enzyme can be dissociated into two subunits: one contains the enzymatic activity and (in the dissociated form) does not bind CTP; the other binds CTP but has no catalytic activity. Apart from providing physical evidence that pacemaker enzymes contain distinct catalytic and regulatory sites, the interaction of aspartate carbamoyltransferase with the different nucleotides provides an explanation for the control of the supply of nucleic acid precursors. If a cell contains sufficient pyrimidine nucleotides (e.g., UTP), aspartate carbamoyltransferase, the first enzyme of pyrimidine biosynthesis, is inhibited. If, however, the cell contains high levels of purine nucleotides (e.g., ATP), as required for the formation of nucleic acids, the inhibition of aspartate carbamoyltransferase is relieved, and pyrimidines are formed.Positive modulation
 Not all pacemaker enzymes are controlled by inhibition of their activity. Instead, some are subject to positive modulation—i.e., the effector is required for the efficient functioning of the enzyme. Such enzymes exhibit little activity in the absence of the appropriate allosteric effector. One instance of positive modulation is the anaplerotic fixation of carbon dioxide onto pyruvate and phosphoenolpyruvate (PEP); this example also illustrates how a metabolic product of one route controls the rate of nutrient flow of another (see Figure 9-->
Not all pacemaker enzymes are controlled by inhibition of their activity. Instead, some are subject to positive modulation—i.e., the effector is required for the efficient functioning of the enzyme. Such enzymes exhibit little activity in the absence of the appropriate allosteric effector. One instance of positive modulation is the anaplerotic fixation of carbon dioxide onto pyruvate and phosphoenolpyruvate (PEP); this example also illustrates how a metabolic product of one route controls the rate of nutrient flow of another (see Figure 9--> ).
).The carboxylation of pyruvate in higher organisms 【50--> 】 and the carboxylation of phosphoenolpyruvate in gut bacteria 【50a-->
】 and the carboxylation of phosphoenolpyruvate in gut bacteria 【50a--> 】 occurs at a significant rate only if acetyl coenzyme A is present. Acetyl coenzyme A acts as a positive allosteric effector and is not broken down in the course of the reaction. Moreover, some pyruvate carboxylases 【50-->
】 occurs at a significant rate only if acetyl coenzyme A is present. Acetyl coenzyme A acts as a positive allosteric effector and is not broken down in the course of the reaction. Moreover, some pyruvate carboxylases 【50--> 】 and the PEP carboxylase of gut bacteria are inhibited by four-carbon compounds (e.g., aspartate). These substances inhibit because they interfere with the binding of the positive effector, acetyl coenzyme A. Such enzymatic controls are reasonable in a physiological sense: it will be recalled that anaplerotic formation of oxaloacetate from pyruvate or PEP is required to provide the acceptor for the entry of acetyl coenzyme A into the TCA cycle. The reaction need occur only if acetyl coenzyme A is present in sufficient amounts. On the other hand, an abundance of four-carbon intermediates obviates the necessity for forming more through carboxylation reactions such as 【50-->
】 and the PEP carboxylase of gut bacteria are inhibited by four-carbon compounds (e.g., aspartate). These substances inhibit because they interfere with the binding of the positive effector, acetyl coenzyme A. Such enzymatic controls are reasonable in a physiological sense: it will be recalled that anaplerotic formation of oxaloacetate from pyruvate or PEP is required to provide the acceptor for the entry of acetyl coenzyme A into the TCA cycle. The reaction need occur only if acetyl coenzyme A is present in sufficient amounts. On the other hand, an abundance of four-carbon intermediates obviates the necessity for forming more through carboxylation reactions such as 【50--> 】 and 【50a-->
】 and 【50a--> 】.
】.
 】 and the carboxylation of phosphoenolpyruvate in gut bacteria 【50a-->
】 and the carboxylation of phosphoenolpyruvate in gut bacteria 【50a--> 】 occurs at a significant rate only if acetyl coenzyme A is present. Acetyl coenzyme A acts as a positive allosteric effector and is not broken down in the course of the reaction. Moreover, some pyruvate carboxylases 【50-->
】 occurs at a significant rate only if acetyl coenzyme A is present. Acetyl coenzyme A acts as a positive allosteric effector and is not broken down in the course of the reaction. Moreover, some pyruvate carboxylases 【50--> 】 and the PEP carboxylase of gut bacteria are inhibited by four-carbon compounds (e.g., aspartate). These substances inhibit because they interfere with the binding of the positive effector, acetyl coenzyme A. Such enzymatic controls are reasonable in a physiological sense: it will be recalled that anaplerotic formation of oxaloacetate from pyruvate or PEP is required to provide the acceptor for the entry of acetyl coenzyme A into the TCA cycle. The reaction need occur only if acetyl coenzyme A is present in sufficient amounts. On the other hand, an abundance of four-carbon intermediates obviates the necessity for forming more through carboxylation reactions such as 【50-->
】 and the PEP carboxylase of gut bacteria are inhibited by four-carbon compounds (e.g., aspartate). These substances inhibit because they interfere with the binding of the positive effector, acetyl coenzyme A. Such enzymatic controls are reasonable in a physiological sense: it will be recalled that anaplerotic formation of oxaloacetate from pyruvate or PEP is required to provide the acceptor for the entry of acetyl coenzyme A into the TCA cycle. The reaction need occur only if acetyl coenzyme A is present in sufficient amounts. On the other hand, an abundance of four-carbon intermediates obviates the necessity for forming more through carboxylation reactions such as 【50--> 】 and 【50a-->
】 and 【50a--> 】.
】. Special Comp-->
Special Comp-->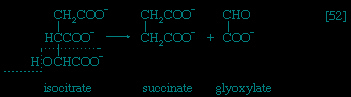 Similar reasoning, though in the opposite sense, can be applied to the control of another anaplerotic sequence, the glyoxylate cycle (Figure 8-->
Similar reasoning, though in the opposite sense, can be applied to the control of another anaplerotic sequence, the glyoxylate cycle (Figure 8--> ). The biosynthesis of cell materials from the two-carbon compound acetate is, in principle, akin to biosynthesis from TCA cycle intermediates. In both processes, it is the availability of intermediates such as PEP and pyruvate that determines the rate at which a cell forms the many components produced through gluconeogenesis. Although in the strictest sense the glyoxylate cycle has no defined end product, PEP and pyruvate are, for these physiological reasons, best fitted to regulate the rate at which the glyoxylate cycle is required to operate. It is thus not unexpected that the pacemaker enzyme of the glyoxylate cycle, isocitrate lyase (reaction 【52-->
). The biosynthesis of cell materials from the two-carbon compound acetate is, in principle, akin to biosynthesis from TCA cycle intermediates. In both processes, it is the availability of intermediates such as PEP and pyruvate that determines the rate at which a cell forms the many components produced through gluconeogenesis. Although in the strictest sense the glyoxylate cycle has no defined end product, PEP and pyruvate are, for these physiological reasons, best fitted to regulate the rate at which the glyoxylate cycle is required to operate. It is thus not unexpected that the pacemaker enzyme of the glyoxylate cycle, isocitrate lyase (reaction 【52-->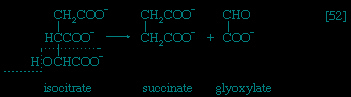 】), is allosterically inhibited by PEP and by pyruvate.
】), is allosterically inhibited by PEP and by pyruvate. energy state of the cell
 It is characteristic of catabolic routes that they do not lead to uniquely identifiable end products. The major products of glycolysis and the TCA cycle, for example, are carbon dioxide and water. Within the cell, the concentrations of both are unlikely to vary sufficiently to allow them to serve as effective regulatory metabolites. The processes by which water is produced (Figure 7-->
It is characteristic of catabolic routes that they do not lead to uniquely identifiable end products. The major products of glycolysis and the TCA cycle, for example, are carbon dioxide and water. Within the cell, the concentrations of both are unlikely to vary sufficiently to allow them to serve as effective regulatory metabolites. The processes by which water is produced (Figure 7--> ) initially involve, however, the reduction of coenzymes, the reoxidation of which is accompanied by the synthesis of ATP from ADP. Moreover, as described in previous sections, the utilization of ATP in energy-consuming reactions yields ADP and AMP. At any given moment, therefore, a living cell contains ATP, ADP, and AMP; the relative proportion of the three nucleotides provides an index of the energy state of the cell. It is thus reasonable that the flux of nutrients through catabolic routes is, in general, impeded by high intracellular levels of both reduced coenzymes (e.g., FADH2, reduced NAD+) and ATP, and that these inhibitory effects are often overcome by AMP.
) initially involve, however, the reduction of coenzymes, the reoxidation of which is accompanied by the synthesis of ATP from ADP. Moreover, as described in previous sections, the utilization of ATP in energy-consuming reactions yields ADP and AMP. At any given moment, therefore, a living cell contains ATP, ADP, and AMP; the relative proportion of the three nucleotides provides an index of the energy state of the cell. It is thus reasonable that the flux of nutrients through catabolic routes is, in general, impeded by high intracellular levels of both reduced coenzymes (e.g., FADH2, reduced NAD+) and ATP, and that these inhibitory effects are often overcome by AMP. The control exerted by the levels of ATP, ADP, and AMP within the cell is illustrated by the regulatory mechanisms of glycolysis and the TCA cycle (Figure 9-->
The control exerted by the levels of ATP, ADP, and AMP within the cell is illustrated by the regulatory mechanisms of glycolysis and the TCA cycle (Figure 9--> ); these nucleotides also serve to govern the occurrence of the opposite pathway, gluconeogenesis, and to avoid mutual interference of the catabolic and anabolic sequences. Although not all of the controls mentioned below have been found to operate in all living organisms examined, it has been observed that, in general:
); these nucleotides also serve to govern the occurrence of the opposite pathway, gluconeogenesis, and to avoid mutual interference of the catabolic and anabolic sequences. Although not all of the controls mentioned below have been found to operate in all living organisms examined, it has been observed that, in general:1. Glucose 6-phosphate stimulates glycogen synthesis from glucose 1-phosphate 【79--> 】 and inhibits both glycogen breakdown 【16-->
】 and inhibits both glycogen breakdown 【16--> 】 and its own formation from glucose 【1-->
】 and its own formation from glucose 【1--> 】.
】.
 】 and inhibits both glycogen breakdown 【16-->
】 and inhibits both glycogen breakdown 【16--> 】 and its own formation from glucose 【1-->
】 and its own formation from glucose 【1--> 】.
】.2. phosphofructokinase, the most important pacemaker enzyme of glycolysis 【3--> 】, is inhibited by high levels of its own substrates (fructose 6-phosphate and ATP); this inhibition is overcome by AMP. In tissues, such as heart muscle, which use fatty acids as a major fuel, inhibition of glycolysis by citrate may be physiologically the more important means of control. Control by citrate, the first intermediate of the TCA cycle, which produces the bulk of the cellular ATP, is thus the same, in principle, as control through ATP.
】, is inhibited by high levels of its own substrates (fructose 6-phosphate and ATP); this inhibition is overcome by AMP. In tissues, such as heart muscle, which use fatty acids as a major fuel, inhibition of glycolysis by citrate may be physiologically the more important means of control. Control by citrate, the first intermediate of the TCA cycle, which produces the bulk of the cellular ATP, is thus the same, in principle, as control through ATP.
 】, is inhibited by high levels of its own substrates (fructose 6-phosphate and ATP); this inhibition is overcome by AMP. In tissues, such as heart muscle, which use fatty acids as a major fuel, inhibition of glycolysis by citrate may be physiologically the more important means of control. Control by citrate, the first intermediate of the TCA cycle, which produces the bulk of the cellular ATP, is thus the same, in principle, as control through ATP.
】, is inhibited by high levels of its own substrates (fructose 6-phosphate and ATP); this inhibition is overcome by AMP. In tissues, such as heart muscle, which use fatty acids as a major fuel, inhibition of glycolysis by citrate may be physiologically the more important means of control. Control by citrate, the first intermediate of the TCA cycle, which produces the bulk of the cellular ATP, is thus the same, in principle, as control through ATP.3. Fructose 1,6-diphosphatase 【59--> 】, which catalyzes the reaction opposite to phosphofructokinase, is strongly inhibited by AMP.
】, which catalyzes the reaction opposite to phosphofructokinase, is strongly inhibited by AMP.
 】, which catalyzes the reaction opposite to phosphofructokinase, is strongly inhibited by AMP.
】, which catalyzes the reaction opposite to phosphofructokinase, is strongly inhibited by AMP.4. Rapid catabolism of carbohydrate requires the efficient conversion of PEP to pyruvate. In liver and in some bacteria the activity of the pyruvate kinase that catalyzes this process 【10--> 】 is greatly stimulated by the presence of fructose 1,6-diphosphate, which thus acts as a potentiator of a reaction required for its ultimate catabolism.
】 is greatly stimulated by the presence of fructose 1,6-diphosphate, which thus acts as a potentiator of a reaction required for its ultimate catabolism.
 】 is greatly stimulated by the presence of fructose 1,6-diphosphate, which thus acts as a potentiator of a reaction required for its ultimate catabolism.
】 is greatly stimulated by the presence of fructose 1,6-diphosphate, which thus acts as a potentiator of a reaction required for its ultimate catabolism.5. The oxidation of pyruvate to acetyl coenzyme A 【37-->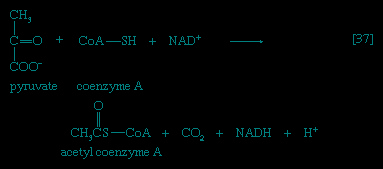 】 is inhibited by acetyl coenzyme A. Because acetyl coenzyme A also acts as a positive modulator of pyruvate carboxylation 【50-->
】 is inhibited by acetyl coenzyme A. Because acetyl coenzyme A also acts as a positive modulator of pyruvate carboxylation 【50--> 】, this control reinforces the partition between pyruvate catabolism and its conversion to four-carbon intermediates for anaplerosis and gluconeogenesis.
】, this control reinforces the partition between pyruvate catabolism and its conversion to four-carbon intermediates for anaplerosis and gluconeogenesis.
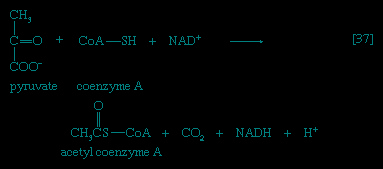 】 is inhibited by acetyl coenzyme A. Because acetyl coenzyme A also acts as a positive modulator of pyruvate carboxylation 【50-->
】 is inhibited by acetyl coenzyme A. Because acetyl coenzyme A also acts as a positive modulator of pyruvate carboxylation 【50--> 】, this control reinforces the partition between pyruvate catabolism and its conversion to four-carbon intermediates for anaplerosis and gluconeogenesis.
】, this control reinforces the partition between pyruvate catabolism and its conversion to four-carbon intermediates for anaplerosis and gluconeogenesis.6. Citrate synthase 【38--> 】, the first enzyme of the TCA cycle, is inhibited by ATP in higher organisms and by reduced NAD+ in many microorganisms. In some strictly aerobic bacteria, the inhibition by reduced NAD+ is overcome by AMP.
】, the first enzyme of the TCA cycle, is inhibited by ATP in higher organisms and by reduced NAD+ in many microorganisms. In some strictly aerobic bacteria, the inhibition by reduced NAD+ is overcome by AMP.
 】, the first enzyme of the TCA cycle, is inhibited by ATP in higher organisms and by reduced NAD+ in many microorganisms. In some strictly aerobic bacteria, the inhibition by reduced NAD+ is overcome by AMP.
】, the first enzyme of the TCA cycle, is inhibited by ATP in higher organisms and by reduced NAD+ in many microorganisms. In some strictly aerobic bacteria, the inhibition by reduced NAD+ is overcome by AMP.7. Citrate acts as a positive effector for the first enzyme of fatty acid biosynthesis 【62--> 】. A high level of citrate, which also indicates a sufficient energy supply, thus inhibits carbohydrate fragmentation (see 【2-->
】. A high level of citrate, which also indicates a sufficient energy supply, thus inhibits carbohydrate fragmentation (see 【2--> 】) and diverts the carbohydrate that has been fragmented from combustion to the formation of lipids.
】) and diverts the carbohydrate that has been fragmented from combustion to the formation of lipids.
 】. A high level of citrate, which also indicates a sufficient energy supply, thus inhibits carbohydrate fragmentation (see 【2-->
】. A high level of citrate, which also indicates a sufficient energy supply, thus inhibits carbohydrate fragmentation (see 【2--> 】) and diverts the carbohydrate that has been fragmented from combustion to the formation of lipids.
】) and diverts the carbohydrate that has been fragmented from combustion to the formation of lipids.8. Some forms of isocitrate dehydrogenase 【40-->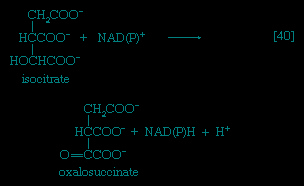 】 are maximally active only in the presence of ADP or AMP and are inhibited by ATP (adenosine triphosphate). This is an example of regulation by covalent modification of an enzyme since the action of ATP here is to phosphorylate, and consequently to inactivate, the isocitrate dehydrogenase. A specific phosphatase, which is a different enzymatic activity of the protein that effects the phosphorylation by ATP, catalyzes the splitting-off by water of the phosphate moiety on the inactive isocitrate dehydrogenase and thus restricts activity. Again, the energy state of the cell serves as the signal regulating an enzyme involved in energy transduction.
】 are maximally active only in the presence of ADP or AMP and are inhibited by ATP (adenosine triphosphate). This is an example of regulation by covalent modification of an enzyme since the action of ATP here is to phosphorylate, and consequently to inactivate, the isocitrate dehydrogenase. A specific phosphatase, which is a different enzymatic activity of the protein that effects the phosphorylation by ATP, catalyzes the splitting-off by water of the phosphate moiety on the inactive isocitrate dehydrogenase and thus restricts activity. Again, the energy state of the cell serves as the signal regulating an enzyme involved in energy transduction.
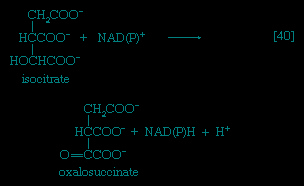 】 are maximally active only in the presence of ADP or AMP and are inhibited by ATP (adenosine triphosphate). This is an example of regulation by covalent modification of an enzyme since the action of ATP here is to phosphorylate, and consequently to inactivate, the isocitrate dehydrogenase. A specific phosphatase, which is a different enzymatic activity of the protein that effects the phosphorylation by ATP, catalyzes the splitting-off by water of the phosphate moiety on the inactive isocitrate dehydrogenase and thus restricts activity. Again, the energy state of the cell serves as the signal regulating an enzyme involved in energy transduction.
】 are maximally active only in the presence of ADP or AMP and are inhibited by ATP (adenosine triphosphate). This is an example of regulation by covalent modification of an enzyme since the action of ATP here is to phosphorylate, and consequently to inactivate, the isocitrate dehydrogenase. A specific phosphatase, which is a different enzymatic activity of the protein that effects the phosphorylation by ATP, catalyzes the splitting-off by water of the phosphate moiety on the inactive isocitrate dehydrogenase and thus restricts activity. Again, the energy state of the cell serves as the signal regulating an enzyme involved in energy transduction.Coarse control
Although fine control mechanisms allow the sensitive adjustment of the flux of nutrients along metabolic pathways relative to the needs of cells under relatively constant environmental conditions, these processes may not be adequate to cope with severe changes in the chemical milieu.
Such severe changes may arise in higher organisms with a change in diet or when, in response to other stimuli, the hormonal balance is altered. In starvation, for example, the overriding need to maintain blood glucose levels may require the liver to synthesize glucose from noncarbohydrate products of tissue breakdown at rates greater than can be achieved by the enzymes normally present in the liver. Under such circumstances, cellular concentrations of key enzymes of gluconeogenesis, such as pyruvate carboxylase 【50--> 】 and PEP carboxykinase 【54-->
】 and PEP carboxykinase 【54-->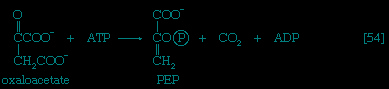 】, may rise by as much as 10-fold, while the concentration of glucokinase 【1-->
】, may rise by as much as 10-fold, while the concentration of glucokinase 【1--> 】 and of the enzymes of fatty acid synthesis decreases to a similar extent. Conversely, high carbohydrate diets and administration of the hormone insulin to diabetic animals elicit a preferential synthesis of glucokinase 【1-->
】 and of the enzymes of fatty acid synthesis decreases to a similar extent. Conversely, high carbohydrate diets and administration of the hormone insulin to diabetic animals elicit a preferential synthesis of glucokinase 【1--> 】 and pyruvate kinase 【10-->
】 and pyruvate kinase 【10--> 】. These changes in the relative proportions and absolute amounts of key enzymes are the net result of increases in the rate of their synthesis and decreases in the rate of their destruction. Although such changes reflect changes in the rates of either transcription, translation, or both of specific regions of the genome, the mechanisms by which the changes are effected have not yet been clarified.
】. These changes in the relative proportions and absolute amounts of key enzymes are the net result of increases in the rate of their synthesis and decreases in the rate of their destruction. Although such changes reflect changes in the rates of either transcription, translation, or both of specific regions of the genome, the mechanisms by which the changes are effected have not yet been clarified.
 】 and PEP carboxykinase 【54-->
】 and PEP carboxykinase 【54-->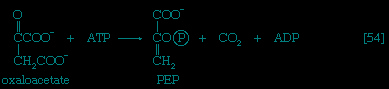 】, may rise by as much as 10-fold, while the concentration of glucokinase 【1-->
】, may rise by as much as 10-fold, while the concentration of glucokinase 【1--> 】 and of the enzymes of fatty acid synthesis decreases to a similar extent. Conversely, high carbohydrate diets and administration of the hormone insulin to diabetic animals elicit a preferential synthesis of glucokinase 【1-->
】 and of the enzymes of fatty acid synthesis decreases to a similar extent. Conversely, high carbohydrate diets and administration of the hormone insulin to diabetic animals elicit a preferential synthesis of glucokinase 【1--> 】 and pyruvate kinase 【10-->
】 and pyruvate kinase 【10--> 】. These changes in the relative proportions and absolute amounts of key enzymes are the net result of increases in the rate of their synthesis and decreases in the rate of their destruction. Although such changes reflect changes in the rates of either transcription, translation, or both of specific regions of the genome, the mechanisms by which the changes are effected have not yet been clarified.
】. These changes in the relative proportions and absolute amounts of key enzymes are the net result of increases in the rate of their synthesis and decreases in the rate of their destruction. Although such changes reflect changes in the rates of either transcription, translation, or both of specific regions of the genome, the mechanisms by which the changes are effected have not yet been clarified.Microorganisms sometimes encounter changes in environment much more severe than those encountered by the cells of tissues and organs, and their responses are correspondingly greater. Mention has already been made of the ability of E. coli to form β-galactosidase when transferred to a medium containing lactose as the sole carbon source; such a transfer may result in an increase of 1,000-fold or more in the cellular concentration of the enzyme. Because this preferential enzyme synthesis is elicited by exposure of the cells to lactose, or to non-metabolizable but chemically similar analogues, and because synthesis ceases as soon as the eliciting agents (inducers) are removed, β-galactosidase is termed an inducible enzyme. It has been established that a regulator gene exists that specifies the amino-acid sequence of a so-called repressor protein (repression), and that the repressor protein binds to a unique portion of the region of DNA concerned with β-galactosidase formation. Under these circumstances the DNA is not transcribed to mRNA, and virtually no enzyme is made. The repressor, however, is an allosteric protein and readily combines with inducers. Such a combination prevents the repressor from binding to DNA and allows transcription and translation of β-galactosidase to proceed.
 Although this mechanism for the specific control of gene activity may not apply to the regulation of all inducible enzymes—for example, those concerned with the utilization of the sugar arabinose—and is not universally applicable to all coarse control processes in all microorganisms, it can explain the manner in which the presence in growth media of at least some cell components represses (i.e., inhibits the synthesis of) enzymes normally involved in the formation of such components by gut bacteria such as E. coli. Although, for example, the bacteria must obviously make amino acids from ammonia if that is the sole source of nitrogen available to them, it would not be necessary for the bacteria to synthesize enzymes required for the formation of amino acids supplied preformed in the medium. Thus, of the three aspartokinases formed by E. coli (Figure 12-->
Although this mechanism for the specific control of gene activity may not apply to the regulation of all inducible enzymes—for example, those concerned with the utilization of the sugar arabinose—and is not universally applicable to all coarse control processes in all microorganisms, it can explain the manner in which the presence in growth media of at least some cell components represses (i.e., inhibits the synthesis of) enzymes normally involved in the formation of such components by gut bacteria such as E. coli. Although, for example, the bacteria must obviously make amino acids from ammonia if that is the sole source of nitrogen available to them, it would not be necessary for the bacteria to synthesize enzymes required for the formation of amino acids supplied preformed in the medium. Thus, of the three aspartokinases formed by E. coli (Figure 12--> ), two are repressed by their end products, methionine and lysine. On the other hand, the third aspartokinase, which (as described above) is inhibited by threonine, is repressed by threonine only if isoleucine is also present. This example of so-called multivalent repression is of obvious physiological utility. It is likely that the amino acids that thus specifically inhibit the synthesis of aspartokinases do so by combining with specific protein repressor molecules; however, whereas the combination of the inducer with the repressor of β-galactosidase inactivates the repressor protein and hence permits synthesis of the enzyme, the repressor proteins for biosynthetic enzymes would not bind to DNA unless they were also combined with the appropriate amino acid. Aspartokinase synthesis would thus occur in the absence of the end-product effectors and not in their presence.
), two are repressed by their end products, methionine and lysine. On the other hand, the third aspartokinase, which (as described above) is inhibited by threonine, is repressed by threonine only if isoleucine is also present. This example of so-called multivalent repression is of obvious physiological utility. It is likely that the amino acids that thus specifically inhibit the synthesis of aspartokinases do so by combining with specific protein repressor molecules; however, whereas the combination of the inducer with the repressor of β-galactosidase inactivates the repressor protein and hence permits synthesis of the enzyme, the repressor proteins for biosynthetic enzymes would not bind to DNA unless they were also combined with the appropriate amino acid. Aspartokinase synthesis would thus occur in the absence of the end-product effectors and not in their presence. Special Comp-->
Special Comp-->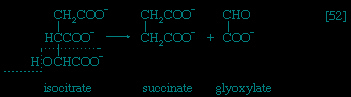 Special Comp-->
Special Comp--> This explanation applies also to the coarse control of the anaplerotic glyoxylate cycle (Figure 8-->
This explanation applies also to the coarse control of the anaplerotic glyoxylate cycle (Figure 8--> ). The synthesis of both of the enzymes unique to that cycle, isocitrate lyase 【52-->
). The synthesis of both of the enzymes unique to that cycle, isocitrate lyase 【52-->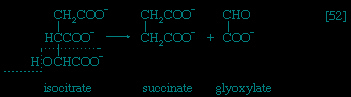 】 and malate synthase 【53-->
】 and malate synthase 【53--> 】, is controlled by a regulator gene that presumably specifies a repressor protein unable to bind to DNA unless combined with pyruvate or PEP. Cells (cell) growing on acetate do not contain high levels of these intermediates because they are continuously being removed for biosynthesis. The enzymes of the glyoxylate cycle are therefore formed at high rates. If pyruvate or substances catabolized to PEP or pyruvate are added to the medium, however, further synthesis of the two enzymes is speedily repressed.
】, is controlled by a regulator gene that presumably specifies a repressor protein unable to bind to DNA unless combined with pyruvate or PEP. Cells (cell) growing on acetate do not contain high levels of these intermediates because they are continuously being removed for biosynthesis. The enzymes of the glyoxylate cycle are therefore formed at high rates. If pyruvate or substances catabolized to PEP or pyruvate are added to the medium, however, further synthesis of the two enzymes is speedily repressed.Additional Reading
General works
Albert L. Lehninger, Principles of Biochemistry (1982); Lubert Stryer, Biochemistry, 2nd ed. (1981); Earlene Brown Cunningham, Biochemistry: Mechanisms of Metabolism (1978); Jay Tepperman and Helen M. Tepperman, Metabolic and Endocrine Physiology: An Introductory Text, 5th ed. (1987); S. Dagley and Donald E. Nicholson, An Introduction to Metabolic Pathways (1970).
Cell metabolism
James Darnell, Harvey Lodish, and David Baltimore, Molecular Cell Biology (1986); T.A.V. Subramanian (ed.), Cell Metabolism, Growth and Environment, 2 vol. (1986); W. Bartley, H.L. Kornberg, and J.R. Quayle (eds.), Essays in Cell Metabolism (1970); J. Frank Henderson and A.R.P. Paterson, Nucleotide Metabolism: An Introduction (1973); David A. Bender, Amino Acid Metabolism, 2nd ed. (1985).
Regulation of metabolism
Daniel E. Atkinson, Cellular Energy Metabolism and Its Regulation (1977); E.A. Newsholme and C. Start, Regulation in Metabolism (1977); Ronald G. Thurman, Frederick C. Kauffman, and Kurt Jungermann (eds.), Regulation of Hepatic Metabolism (1986); and Charles Zapsalis and R. Anderle Beck, Food Chemistry and Nutritional Biochemistry (1985).
- Shirley MacLaine
- Shirley Strickland de la Hunty
- Shirley Temple
- Shirley, William
- shirt
- Shirvan rug
- Shishakli, Adib al-
- Shishaku Ishii Kikujirō
- Shishaku Saitō Makoto
- Shishaku Shibusawa Eiichi
- shish kebab
- Shishkov, Aleksandr Semyonovich
- Shitao
- Shiva
- shivah
- Shiv Narayan Agnihotri
- Shivpuri
- Shivpuri National Park
- Shiyali Ramamrita Ranganathan
- Shizuoka
- Shklovsky, Viktor Borisovich
- Shkodër
- S.H. Kress
- Shlisselburg
- Shlonsky, Abraham