Names of fundamental structures most often used in steroid nomenclature
Table
Names of fundamental structures most often used in steroid nomenclature
carbon atoms present (as numbered in structure 6) naturally occurring
general
classes examples shown in text
gonane 1–17 none gonane* (1)
estrane 1–18 estrogens estradiol (17f)
androstane 1–19 androgens androstane* (2–5); testosterone (17e); androstanedione (17c)
pregnane 1–21 gestogens and adrenal steroids progesterone (17a); cortisol (17b); aldosterone (17d); batrachotoxin (14)
cholane 1–24 bile acids cholic acid (10a); sodium sodium glycocholate (10b)
cholestane 1–27 sterols cholesterol (16d); scymnol (11a)
ergostane 1–28 sterols ergosterol (8); cyasterone (22)
stigmastane 1–29 sterols stigmasterol (7); antheridiol (12)
lanostane 1–27; 30–32 trimethyl sterols lanosterol (16c);
22,25-oxidoholothurinogenin (13)
cardanolide 1–23 cardiac glycosides digitoxigenin (23)
bufanolide 1–24 toad poisons bufotoxin (24)
spirostan 1–27 sapogenins dioscin (25)
*Gonane and androstane themselves do not occur in nature.
See as table: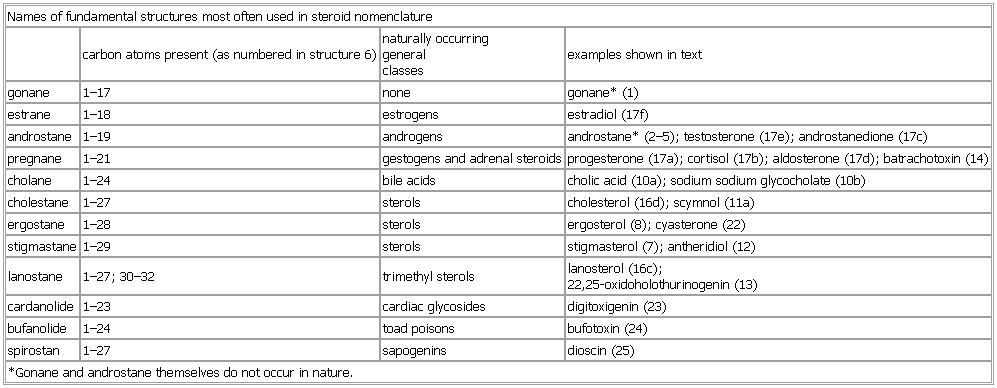

- crop rotation
- croquet
- Crosby, Bing
- Crosby, Fanny
- Crosby, Harry
- Crosby, Sidney
- Crosby, Stills and Nash
- Cros, Charles
- crosier
- cross
- crossbill
- crossbow
- cross-country
- cross-country skiing
- cross-cousin
- Crossett
- cross-fertilization
- Cross, Hardy
- Cross of Gold speech
- crossopterygian
- Crossosomatales
- cross ratio
- Cross, Richard Assheton Cross, 1st Viscount
- Cross River
- cross section