neodymium
chemical element
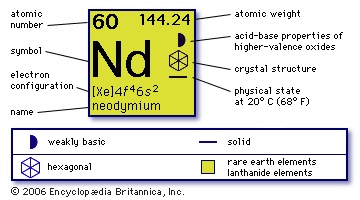 (Nd), chemical element, rare-earth metal of the lanthanoid series of the periodic table. Neodymium is silvery-white in colour and tarnishes in air to form an oxide which chips, exposing the metal to further oxidation. The metal must be sealed in a plastic covering or kept in mineral oil for preservation. It reacts gradually with cold water and rapidly with hot water to liberate hydrogen. Carl Auer von Welsbach (Welsbach, Carl Auer, Freiherr von) discovered neodymium (1885) by separating ammonium didymium nitrate prepared from didymia (a mixture of rare-earth oxides) into a neodymium fraction and a praseodymium fraction by repeated crystallization. Of the rare earths, only cerium and yttrium are more plentiful than neodymium. In the igneous rocks of the Earth's crust it is more than twice as abundant as lead and about half as plentiful as copper. Neodymium occurs in the minerals monazite and bastnaesite and is a product of nuclear fission. Ion-exchange techniques have supplanted fractional crystallization for separation and purification of neodymium. The metal itself is prepared by electrolysis of the fused halides or by thermoreduction of the fluoride with calcium or lithium.
(Nd), chemical element, rare-earth metal of the lanthanoid series of the periodic table. Neodymium is silvery-white in colour and tarnishes in air to form an oxide which chips, exposing the metal to further oxidation. The metal must be sealed in a plastic covering or kept in mineral oil for preservation. It reacts gradually with cold water and rapidly with hot water to liberate hydrogen. Carl Auer von Welsbach (Welsbach, Carl Auer, Freiherr von) discovered neodymium (1885) by separating ammonium didymium nitrate prepared from didymia (a mixture of rare-earth oxides) into a neodymium fraction and a praseodymium fraction by repeated crystallization. Of the rare earths, only cerium and yttrium are more plentiful than neodymium. In the igneous rocks of the Earth's crust it is more than twice as abundant as lead and about half as plentiful as copper. Neodymium occurs in the minerals monazite and bastnaesite and is a product of nuclear fission. Ion-exchange techniques have supplanted fractional crystallization for separation and purification of neodymium. The metal itself is prepared by electrolysis of the fused halides or by thermoreduction of the fluoride with calcium or lithium.The metal is used in the electronics industry, in the manufacture of steel, and as a component in a number of alloys, among them misch metal (15 percent neodymium), used for cigarette-lighter flints. Alloyed with iron and boron, neodymium is the basis for powerful permanent magnets used in computer hard drives, lightweight earphones, and numerous other applications. Its compounds are used in the ceramics industry for glazes and to colour glass. The crude oxide Nd2O3 is used to counteract the green colour of ferrous compounds in glass; and the more pure compound is used in the production of the only known glass that is bright purple in colour. This neodymium glass can be used instead of ruby as a laser material. A mixture of neodymium and praseodymium absorbs light in the region of the harmful sodium-D (spectral) lines and therefore is used in the glass of welders' and glassblowers' goggles.
Natural neodymium is a mixture of seven different isotopes: neodymium-142 (27.1 percent), neodymium-144 (23.8 percent), neodymium-146 (17.2 percent), neodymium-143 (12.2 percent), neodymium-145 (8.3 percent), neodymium-148 (5.8 percent), and neodymium-150 (5.6 percent). All are stable except the weakly radioactive neodymium-144, the lightest natural nuclide that decays by alpha emission. Two allotropes (structural forms) exist; at room temperature the structure is hexagonal close-packed. The element in the +3 oxidation state forms compounds such as the oxide Nd2O3 and the hydroxide Nd(OH)3; the Nd3+ ion is stable in water. A few compounds of neodymium in the +2 state have been prepared such as the diiodide NdI2, and the dichloride NdCl2; the Nd2+ ion is unstable in aqueous solution.
atomic number
60
atomic weight
144.240
melting point
1,021° C (1,870° F)
boiling point
3,068° C (5,554° F)
specific gravity
7.007 (25° C)
oxidation states
+2, +3
electronic config.
【Xe】4f45d06s2
- Cocoa-Rockledge
- Coco Chanel
- coco de mer
- Coconuco
- coconut palm
- cocoon
- coco plum
- Coco River
- Cocos Island
- Cocos Islands
- Cocteau, Jean
- cod
- coda
- Cod, Cape
- Coddington, William
- code
- codeine
- Code of Canon Law
- Code of Hammurabi
- Code of Holiness
- Code of Huesca
- Code of Justinian
- Code of Kalantiyaw
- codependency
- codex