nuclear fusion
physics
Introduction
 process by which nuclear reactions between light elements form heavier elements (up to iron). In cases where the interacting nuclei belong to elements with low atomic numbers (atomic number) (e.g., hydrogen 【atomic number 1】 or its isotopes deuterium and tritium), substantial amounts of energy are released. The vast energy potential of nuclear fusion was first exploited in thermonuclear weapons, or hydrogen bombs, which were developed in the decade immediately following World War II. For a detailed history of this development, see nuclear weapon. Meanwhile, the potential peaceful applications of nuclear fusion, especially in view of the essentially limitless supply of fusion fuel on Earth, have encouraged an immense effort to harness this process for the production of power. For more detailed information on this effort, see fusion reactor.
process by which nuclear reactions between light elements form heavier elements (up to iron). In cases where the interacting nuclei belong to elements with low atomic numbers (atomic number) (e.g., hydrogen 【atomic number 1】 or its isotopes deuterium and tritium), substantial amounts of energy are released. The vast energy potential of nuclear fusion was first exploited in thermonuclear weapons, or hydrogen bombs, which were developed in the decade immediately following World War II. For a detailed history of this development, see nuclear weapon. Meanwhile, the potential peaceful applications of nuclear fusion, especially in view of the essentially limitless supply of fusion fuel on Earth, have encouraged an immense effort to harness this process for the production of power. For more detailed information on this effort, see fusion reactor.This article focuses on the physics of the fusion reaction and on the principles of achieving sustained energy-producing fusion reactions.
The fusion reaction
Fusion reactions constitute the fundamental energy source of stars, including the Sun. The evolution of stars can be viewed as a passage through various stages as thermonuclear reactions and nucleosynthesis cause compositional changes over long time spans. hydrogen (H) “burning” initiates the fusion energy source of stars and leads to the formation of helium (He). Generation of fusion energy for practical use also relies on fusion reactions between the lightest elements that burn to form helium. In fact, the heavy isotopes of hydrogen— deuterium (D) and tritium (T)—react more efficiently with each other, and, when they do undergo fusion, they yield more energy per reaction than do two hydrogen nuclei. (The hydrogen nucleus consists of a single proton. The deuterium nucleus has one proton and one neutron, while tritium has one proton and two neutrons.)
Fusion reactions between light elements, like fission reactions that split heavy elements, release energy because of a key feature of nuclear matter called the binding energy, which can be released through fusion or fission. The binding energy of the nucleus is a measure of the efficiency with which its constituent nucleons are bound together. Take, for example, an element with Z protons and N neutrons in its nucleus. The element's atomic weight A is Z+N, and its atomic number is Z. The binding energy B is the energy associated with the mass difference between the Z protons and N neutrons considered separately and the nucleons bound together (Z+N) in a nucleus of mass M. The formula is
B=(Zmp+Nmn−M)c2,
where mp and mn are the proton and neutron masses and c is the speed of light. It has been determined experimentally that the binding energy per nucleon is a maximum of about 1.410−12 joule at an atomic mass number of approximately 60—that is, approximately the atomic mass number of iron. Accordingly, the fusion of elements lighter than iron or the splitting of heavier ones generally leads to a net release of energy.
Two types of fusion reactions
Fusion reactions are of two basic types: (1) those that preserve the number of protons and neutrons and (2) those that involve a conversion between protons and neutrons. Reactions of the first type are most important for practical fusion energy production, whereas those of the second type are crucial to the initiation of star burning. An arbitrary element is indicated by the notation AZX, where Z is the charge of the nucleus and A is the atomic weight. An important fusion reaction for practical energy generation is that between deuterium and tritium (the D-T fusion reaction). It produces helium (He) and a neutron (n) and is written
D+T→He+n.
To the left of the arrow (before the reaction) there are two protons and three neutrons. The same is true on the right.
The other reaction, that which initiates star burning, involves the fusion of two hydrogen nuclei to form deuterium (the H-H fusion reaction):
H+H→D+β++ν,
where β+ represents a positron and ν stands for a neutrino. Before the reaction there are two hydrogen nuclei (that is, two protons). Afterward there are one proton and one neutron (bound together as the nucleus of deuterium) plus a positron and a neutrino (produced as a consequence of the conversion of one proton to a neutron).
Both of these fusion reactions are exoergic and so yield energy. The German-born physicist Hans Bethe (Bethe, Hans) proposed in the 1930s that the H-H fusion reaction could occur with a net release of energy and provide, along with subsequent reactions, the fundamental energy source sustaining the stars. However, practical energy generation requires the D-T reaction for two reasons: first, the rate of reactions between deuterium and tritium is much higher than that between protons; second, the net energy release from the D-T reaction is 40 times greater than that from the H-H reaction.
energy released in fusion reactions
Energy is released in a nuclear reaction if the total mass of the resultant particles is less than the mass of the initial reactants. To illustrate, suppose two nuclei, labeled X and a, react to form two other nuclei, Y and b, denoted
X+a→Y+b.
The particles a and b are often nucleons, either protons or neutrons, but in general can be any nuclei. Assuming that none of the particles is internally excited (i.e., each is in its ground state), the energy quantity called the Q-value for this reaction is defined as
Q=(mx+ma−mb−my)c2,
where the m-letters refer to the mass of each particle and c is the speed of light. When the energy value Q is positive, the reaction is exoergic; when Q is negative, the reaction is endoergic (i.e., absorbs energy). When both the total proton number and the total neutron number are preserved before and after the reaction (as in D-T reactions), then the Q-value can be expressed in terms of the binding energy B of each particle as
Q=By+Bb−Bx−Ba.
The D-T fusion reaction has a positive Q-value of 2.8×10−12 joule. The H-H fusion reaction is also exoergic, with a Q-value of 6.7×10−14 joule. To develop a sense for these figures, one might consider that one metric ton (1,000 kg, or almost 2,205 pounds) of deuterium would contain roughly 3×1032 atoms. If one ton of deuterium were to be consumed through the fusion reaction with tritium, the energy released would be 8.4×1020 joules. This can be compared with the energy content of one ton of coal—namely, 2.9×1010 joules. In other words, one ton of deuterium has the energy equivalent of approximately 29 billion tons of coal.
Rate and yield of fusion reactions
The energy yield of a reaction between nuclei and the rate of such reactions are both important. These quantities have a profound influence in scientific areas such as nuclear astrophysics and the potential for nuclear production of electrical energy.
When a particle of one type passes through a collection of particles of the same or different type, there is a measurable chance that the particles will interact. The particles may interact in many ways, such as simply scattering, which means that they change direction and exchange energy, or they may undergo a nuclear fusion reaction. The measure of the likelihood that particles will interact is called the cross section, and the magnitude of the cross section depends on the type of interaction and the state and energy of the particles. The product of the cross section and the atomic density of the target particle is called the macroscopic cross section. The inverse of the macroscopic cross section is particularly noteworthy as it gives the mean distance an incident particle will travel before interacting with a target particle; this inverse measure is called the mean free path. Cross sections are measured by producing a beam of one particle at a given energy, allowing the beam to interact with a (usually thin) target made of the same or a different material, and measuring deflections or reaction products. In this way it is possible to determine the relative likelihood of one type of fusion reaction versus another, as well as the optimal conditions for a particular reaction.
The cross sections of fusion reactions can be measured experimentally or calculated theoretically, and they have been determined for many reactions over a wide range of particle energies. They are well known for practical fusion energy applications and are reasonably well known, though with gaps, for stellar evolution. Fusion reactions between nuclei, each with a positive charge of one or more, are the most important for both practical applications and the nucleosynthesis of the light elements in the burning stages of stars. Yet, it is well known that two positively charged nuclei repel each other electrostatically—i.e., they experience a repulsive force inversely proportional to the square of the distance separating them. This repulsion is called the Coulomb barrier (see Coulomb force). It is highly unlikely that two positive nuclei will approach each other closely enough to undergo a fusion reaction unless they have sufficient energy to overcome the Coulomb barrier. As a result, the cross section for fusion reactions between charged particles is very small unless the energy of the particles is high, at least 104 electron volts (electron volt) (1eV1.602×10−19 joule) and often more than 105 or 106 eV. This explains why the centre of a star must be hot for the fuel to burn and why fuel for practical fusion energy systems must be heated to at least 50,000,000 kelvins (K; 90,000,000 °F). Only then will a reasonable fusion reaction rate and power output be achieved.
 The phenomenon of the Coulomb barrier also explains a fundamental difference between energy generation by nuclear fusion and nuclear fission. While fission of heavy elements can be induced by either protons or neutrons, generation of fission energy for practical applications is dependent on neutrons to induce fission reactions in uranium or plutonium. Having no electric charge, the neutron is free to enter the nucleus even if its energy corresponds to room temperature. Fusion energy, relying as it does on the fusion reaction between light nuclei, occurs only when the particles are sufficiently energetic to overcome the Coulomb repulsive force. This requires the production and heating of the gaseous reactants to the high temperature state known as the plasma state.
The phenomenon of the Coulomb barrier also explains a fundamental difference between energy generation by nuclear fusion and nuclear fission. While fission of heavy elements can be induced by either protons or neutrons, generation of fission energy for practical applications is dependent on neutrons to induce fission reactions in uranium or plutonium. Having no electric charge, the neutron is free to enter the nucleus even if its energy corresponds to room temperature. Fusion energy, relying as it does on the fusion reaction between light nuclei, occurs only when the particles are sufficiently energetic to overcome the Coulomb repulsive force. This requires the production and heating of the gaseous reactants to the high temperature state known as the plasma state.The plasma state (plasma)
Typically, a plasma is a gas that has had some substantial portion of its constituent atoms or molecules ionized by the dissociation of one or more of their electrons. These free electrons enable plasmas to conduct electric charges, and a plasma is the only state of matter in which thermonuclear reactions can occur in a self-sustaining manner. Astrophysics and magnetic fusion research, among other fields, require extensive knowledge of how gases behave in the plasma state. The stars, the solar wind, and much of interstellar space are examples where the matter present is in the plasma state. Very high-temperature plasmas are fully ionized gases, which means that the ratio of neutral gas atoms to charged particles is small. For example, the ionization energy of hydrogen is 13.6 eV, while the average energy of a hydrogen ion in a plasma at 50,000,000 K is 6,462 eV. Thus, essentially all of the hydrogen in this plasma would be ionized.
 A reaction-rate parameter more appropriate to the plasma state is obtained by accounting for the fact that the particles in a plasma, as in any gas, have a distribution of energies. That is to say, not all particles have the same energy. In simple plasmas this energy distribution is given by the Maxwell-Boltzmann distribution law, and the temperature of the gas or plasma is, within a proportionality constant, two-thirds of the average particle energy; i.e., the relationship between the average energy E and temperature T is E = 3kT/2, where k is the Boltzmann constant, 8.62×10−5 eV per kelvin. The intensity of nuclear fusion reactions in a plasma is derived by averaging the product of the particles' speed and their cross sections over a distribution of speeds corresponding to a Maxwell-Boltzmann distribution. The cross section for the reaction depends on the energy or speed of the particles. The averaging process yields a function for a given reaction that depends only on the temperature and can be denoted f(T). The rate of energy released (i.e., the power released) in a reaction between two species, a and b, is
A reaction-rate parameter more appropriate to the plasma state is obtained by accounting for the fact that the particles in a plasma, as in any gas, have a distribution of energies. That is to say, not all particles have the same energy. In simple plasmas this energy distribution is given by the Maxwell-Boltzmann distribution law, and the temperature of the gas or plasma is, within a proportionality constant, two-thirds of the average particle energy; i.e., the relationship between the average energy E and temperature T is E = 3kT/2, where k is the Boltzmann constant, 8.62×10−5 eV per kelvin. The intensity of nuclear fusion reactions in a plasma is derived by averaging the product of the particles' speed and their cross sections over a distribution of speeds corresponding to a Maxwell-Boltzmann distribution. The cross section for the reaction depends on the energy or speed of the particles. The averaging process yields a function for a given reaction that depends only on the temperature and can be denoted f(T). The rate of energy released (i.e., the power released) in a reaction between two species, a and b, isPab=nanbfab(T)Uab,
where na and nb are the density of species a and b in the plasma, respectively, and Uab is the energy released each time a and b undergo a fusion reaction. The parameter Pab properly takes into account both the rate of a given reaction and the energy yield per reaction (see figure--> ).
).
 ).
).Fusion reactions in stars (star)
Fusion reactions are the primary energy source of stars and the mechanism for the nucleosynthesis of the light elements. In the late 1930s Hans Bethe (Bethe, Hans) first recognized that the fusion of hydrogen nuclei to form deuterium is exoergic (i.e., there is a net release of energy) and, together with subsequent nuclear reactions, leads to the synthesis of helium. The formation of helium is the main source of energy emitted by normal stars, such as the Sun, where the burning-core plasma has a temperature of less than 15,000,000 K. However, because the gas from which a star is formed often contains some heavier elements, notably carbon (C) and nitrogen (N), it is important to include nuclear reactions between protons and these nuclei. The reaction chain between protons that ultimately leads to helium is the proton-proton cycle. When protons also induce the burning of carbon and nitrogen, the CN cycle must be considered; and, when oxygen (O) is included, still another alternative scheme, the CNO bi-cycle, must be accounted for. (See carbon cycle.)
The proton-proton (proton-proton cycle) nuclear fusion cycle in a star containing only hydrogen begins with the reaction
H+H→D+β++ν;Q=1.44MeV,
where the Q-value assumes annihilation of the positron by an electron. The deuterium could react with other deuterium nuclei, but, because there is so much hydrogen, the D/H ratio is held to very low values, typically 10−18. Thus, the next step is
H+D→3He+γ;Q=5.49MeV,
where γ indicates that gamma rays (gamma ray) carry off some of the energy yield. The burning of the helium-3 isotope then gives rise to ordinary helium and hydrogen via the last step in the chain:
3He+3He→4He+2(H);Q=12.86MeV.
At equilibrium, helium-3 burns predominantly by reactions with itself because its reaction rate with hydrogen is small, while burning with deuterium is negligible due to the very low deuterium concentration. Once helium-4 builds up, reactions with helium-3 can lead to the production of still-heavier elements, including beryllium-7, beryllium-8, lithium-7, and boron-8, if the temperature is greater than about 10,000,000 K.
The stages of stellar evolution are the result of compositional changes over very long periods. The size of a star, on the other hand, is determined by a balance between the pressure exerted by the hot plasma and the gravitational force of the star's mass. The energy of the burning core is transported toward the surface of the star, where it is radiated at an effective temperature. The effective temperature of the Sun's surface is about 6,000 K, and significant amounts of radiation in the visible and infrared wavelength ranges are emitted.
Fusion reactions for controlled power generation
Reactions between deuterium and tritium are the most important fusion reactions for controlled power generation because the cross sections for their occurrence are high, the practical plasma temperatures required for net energy release are moderate, and the energy yield of the reactions are high—17.58 MeV for the basic D-T fusion reaction.
It should be noted that any plasma containing deuterium automatically produces some tritium and helium-3 from reactions of deuterium with other deuterium ions. Other fusion reactions involving elements with an atomic number above 2 can be used, but only with much greater difficulty. This is because the Coulomb barrier increases with increasing charge of the nuclei, leading to the requirement that the plasma temperature exceed 1,000,000,000 K if a significant rate is to be achieved. Some of the more interesting reactions are:
● H+11B→3(4He);Q=8.68MeV;
● H+6Li→3He+4He;Q=4.023MeV;
● 3He+6Li→H+2(4He);Q=16.88MeV; and
● 3He+6Li→D+7Be;Q=0.113MeV.
Reaction (2) converts lithium-6 to helium-3 and ordinary helium. Interestingly, if reaction (2) is followed by reaction (3), then a proton will again be produced and be available to induce reaction (2), thereby propagating the process. Unfortunately, it appears that reaction (4) is 10 times more likely to occur than reaction (3).
Methods of achieving fusion energy
Practical efforts to harness fusion energy involve two basic approaches to containing a high-temperature plasma of elements that undergo nuclear fusion reactions: magnetic confinement and inertial confinement. A much less likely but nevertheless interesting approach is based on fusion catalyzed by muons (muon); research on this topic is of intrinsic interest in nuclear physics. These three methods are described in some detail in this section. In addition, the processes popularly dubbed cold fusion and bubble fusion are briefly described.
Magnetic confinement
In magnetic confinement the particles and energy of a hot plasma are held in place using magnetic fields (magnetic field). A charged particle in a magnetic field experiences a Lorentz force (magnetism) that is proportional to the product of the particle's velocity and the magnetic field. This force causes electrons (electron) and ions (ion) to spiral about the direction of the magnetic line of force, thereby confining the particles. When the topology of the magnetic field yields an effective magnetic well and the pressure balance between the plasma and the field is stable, the plasma can be confined away from material boundaries. Heat and particles are transported both along and across the field, but energy losses can be prevented in two ways. The first is to increase the strength of the magnetic field at two locations along the field line. Charged particles contained between these points can be made to reflect back and forth, an effect called magnetic mirroring (magnetic mirror). In a basically straight system with a region of intensified magnetic field at each end, particles can still escape through the ends due to scattering between particles as they approach the mirroring points. Such end losses can be avoided altogether by creating a magnetic field in the topology of a torus (i.e., configuration of a doughnut or inner tube).
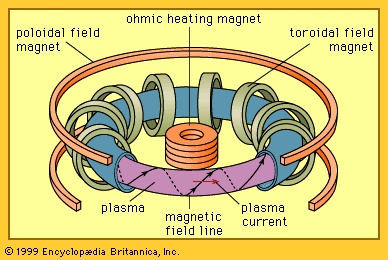 External magnets can be arranged to create a magnetic field topology for stable plasma confinement, or they can be used in conjunction with magnetic fields generated by currents induced to flow in the plasma itself. The late 1960s witnessed a major advance by the Soviet Union in harnessing fusion reactions for practical energy production. Soviet scientists achieved a high plasma temperature (about 3,000,000 K), along with other physical parameters, in a machine referred to as a tokamak (see figure-->
External magnets can be arranged to create a magnetic field topology for stable plasma confinement, or they can be used in conjunction with magnetic fields generated by currents induced to flow in the plasma itself. The late 1960s witnessed a major advance by the Soviet Union in harnessing fusion reactions for practical energy production. Soviet scientists achieved a high plasma temperature (about 3,000,000 K), along with other physical parameters, in a machine referred to as a tokamak (see figure--> ). A tokamak is a toroidal magnetic confinement system in which the plasma is kept stable both by an externally generated, doughnut-shaped magnetic field and by electric currents flowing within the plasma. Since the late 1960s the tokamak has been the major focus of magnetic fusion research worldwide, though other approaches such as the stellarator, the compact torus, and the reversed field pinch (RFP) have also been pursued. In these approaches, the magnetic field lines follow a helical, or screwlike, path as the lines of magnetic force proceed around the torus. In the tokamak the pitch of the helix is weak, so the field lines wind loosely around the poloidal direction (through the central hole) of the torus. In contrast, RFP field lines wind much tighter, wrapping many times in the poloidal direction before completing one loop in the toroidal direction (around the central hole).
). A tokamak is a toroidal magnetic confinement system in which the plasma is kept stable both by an externally generated, doughnut-shaped magnetic field and by electric currents flowing within the plasma. Since the late 1960s the tokamak has been the major focus of magnetic fusion research worldwide, though other approaches such as the stellarator, the compact torus, and the reversed field pinch (RFP) have also been pursued. In these approaches, the magnetic field lines follow a helical, or screwlike, path as the lines of magnetic force proceed around the torus. In the tokamak the pitch of the helix is weak, so the field lines wind loosely around the poloidal direction (through the central hole) of the torus. In contrast, RFP field lines wind much tighter, wrapping many times in the poloidal direction before completing one loop in the toroidal direction (around the central hole).Magnetically confined plasma must be heated to temperatures at which nuclear fusion is vigorous, typically greater than 75,000,000 K (equivalent to an energy of 4,400 eV). This can be achieved by coupling radio-frequency waves or microwaves (microwave) to the plasma particles, by injecting energetic beams of neutral atoms that become ionized and heat the plasma, by magnetically compressing the plasma, or by the ohmic heating (also known as Joule heating) that occurs when an electric current passes through the plasma.
Employing the tokamak concept, scientists and engineers in the United States, Europe, and Japan began in the mid-1980s to use large experimental tokamak devices to attain conditions of temperature, density, and energy confinement that now match those necessary for practical fusion power generation. The machines employed to achieve these results include the Joint European Torus (JET) of the European Union, the Japanese Tokamak-60 (JT-60), and, until 1997, the Tokamak Fusion Test Reactor (TFTR) in the United States. Indeed, in both the TFTR and the JET devices, experiments using deuterium and tritium produced more than 10 megawatts of fusion power and essentially energy breakeven conditions in the plasma itself. Plasma conditions approaching those achieved in tokamaks were also achieved in large stellarator machines in Germany and Japan during the 1990s.
Inertial confinement fusion (ICF)
In this approach, a fuel mass is compressed rapidly to densities 1,000 to 10,000 times greater than normal by generating a pressure as high as 1017 pascals (1012 atmospheres) for periods as short as a nanosecond (10−9 second). Near the end of this time period, the implosion speed exceeds about 3×105 metres per second. At maximum compression of the fuel, which is now in a cool plasma state, the energy in converging shock waves is sufficient to heat the very centre of the fuel to temperatures high enough to induce fusion reactions (greater than an equivalent energy of about 4,400 eV). If the mass of this highly compressed fuel material is large enough, energy will be generated through fusion reactions before this hot plasma ball disassembles. Under proper conditions, much more energy can be released than is required to compress and shock heat the fuel to thermonuclear burning conditions.
The physical processes in ICF bear a relationship to those in thermonuclear weapons and in star formation—namely, collapse, compression heating, and the onset of nuclear fusion. The situation in star formation differs in one respect: gravity is the cause of the collapse, and a collapsed star begins to expand again due to heat from exoergic nuclear fusion reactions. The expansion is ultimately arrested by the gravitational force associated with the enormous mass of the star, at which point a state of equilibrium in both size and temperature is achieved. In contrast, the fuel in a thermonuclear weapon or ICF completely disassembles. In the ideal ICF case, however, this does not occur until about 30 percent of the fusion fuel has burned.
Over the decades, very significant progress has been made in developing the technology and systems for high-energy, short-time-pulse drivers that are necessary to implode the fusion fuel. The most common driver is a high-power laser, though particle accelerators capable of producing beams of high-energy ions are also used. Lasers that produce more than 100,000 joules in pulses of about one nanosecond are now used in experiments, and the power available in short bursts exceeds 1014 watts.
 Two lasers capable of delivering up to 5,000,000 joules in equally short bursts, generating a power level on the fusion targets in excess of 5×1014 watts, are operational. One facility is the Laser MegaJoule in Bordeaux, France. The other is the National Ignition Facility at the Lawrence Livermore National Laboratory in Livermore, Calif., U.S.
Two lasers capable of delivering up to 5,000,000 joules in equally short bursts, generating a power level on the fusion targets in excess of 5×1014 watts, are operational. One facility is the Laser MegaJoule in Bordeaux, France. The other is the National Ignition Facility at the Lawrence Livermore National Laboratory in Livermore, Calif., U.S.Muon-catalyzed fusion
The need in traditional schemes of nuclear fusion to confine very high-temperature plasmas has led some researchers to explore alternatives that would permit fusion reactants to approach each other more closely at much lower temperatures. One method involves substituting muons (muon) (μ) for the electrons that ordinarily surround the nucleus of a fuel atom. Muons are negatively charged subatomic particles (subatomic particle) similar to electrons, except that their mass is a little more than 200 times the electron mass and they are unstable, having a half-life of about 2.2×10−6 second. In fact, fusion has been observed in liquid and gas mixtures of deuterium and tritium at cryogenic (cryogenics) temperatures when muons were injected into the mixture.
Muon-catalyzed fusion is the name given to the process of achieving fusion reactions by causing a deuteron (deuterium nucleus, D+), a triton (tritium nucleus, T+), and a muon to form what is called a muonic molecule. Once a muonic molecule is formed, the rate of fusion reactions is approximately 3×10−8 second. However, the formation of a muonic molecule is complex, involving a series of atomic, molecular, and nuclear processes.
In schematic terms, when a muon enters a mixture of deuterium and tritium, the muon is first captured by one of the two hydrogen isotopes in the mixture, forming either atomic D+-μ or T+-μ, with the atom now in an excited state. The excited atom relaxes to the ground state through a cascade collision process, in which the muon may be transferred from a deuteron to a triton or vice versa. More important, it is also possible that a muonic molecule (D+-μ-T+) will be formed. Although a much rarer reaction, once a muonic molecule does form, fusion takes place almost immediately, releasing the muon in the mixture to be captured again by a deuterium or tritium nucleus and allowing the process to continue. In this sense the muon acts as a catalyst for fusion reactions within the mixture. The key to practical energy production is to generate enough fusion reactions before the muon decays.
The complexities of muon-catalyzed fusion are many and include generating the muons (at an energy expenditure of about five billion electron volts per muon) and immediately injecting them into the deuterium-tritium mixture. In order to produce more energy than what is required to initiate the process, about 300 D-T fusion reactions must take place within the half-life of a muon.
Cold fusion and bubble fusion
Two disputed fusion experiments merit mention. In 1989 two chemists, Martin Fleischmann of the University of Utah and Stanley Pons of the University of Southampton in England, announced that they had produced fusion reactions at essentially room temperature. Their system consisted of electrolytic cells containing heavy water (deuterium oxide, D2O) and palladium rods that absorbed the deuterium from the heavy water. Efforts to give a theoretical explanation of the results failed, as did worldwide efforts to reproduce the claimed cold fusion.
In 2002 Rusi Taleyarkhan and colleagues at Purdue University in Lafayette, Ind., claimed to have observed a statistically significant increase in nuclear emissions of products of fusion reactions (neutrons and tritium) during acoustic cavitation experiments with chilled deuterated (bombarded with deuterium) acetone. Their experimental setup was based on the known phenomenon of sonoluminescence. In sonoluminescence a gas bubble is imploded with high-pressure sound waves. At the end of the implosion process, and for a short time afterward, conditions of high density and temperature are achieved that lead to light emission. By starting with larger, millimetre-sized cavitations (bubbles) that had been deuterated in the acetone liquid, the researchers claimed to have produced densities and temperatures sufficient to induce fusion reactions just before the bubbles broke up. As with cold fusion, most attempts to replicate their results have failed.
Conditions for practical fusion yield
Two conditions must be met to achieve practical energy yields from fusion. First, the plasma temperature must be high enough that fusion reactions occur at a sufficient rate. Second, the plasma must be confined so that the energy released by fusion reactions, when deposited in the plasma, maintains its temperature against loss of energy by such phenomena as conduction, convection, and radiation. When these conditions are achieved, the plasma is said to be ignited. In the case of stars, or some approaches to fusion by magnetic confinement, a steady state can be achieved, and no energy beyond what is supplied from fusion reactions is needed to sustain the system. In other cases, such as the ICF approach, there is a large temperature excursion once fuel ignition is achieved. The energy yield can far exceed the energy required to attain plasma ignition conditions, but this energy is released in a burst, and the process has to be repeated roughly once every second for practical power to be produced.
The conditions for plasma ignition are readily derived. When fusion reactions occur in a plasma, the power released is proportional to the square of plasma ion density, n2. The plasma loses energy when electrons scatter from positively charged ions, accelerating and radiating in the process. Such radiation is called bremsstrahlung and is proportional to n2T1/2, where T is the plasma temperature. Other mechanisms by which heat can escape the plasma lead to a characteristic energy-loss time denoted by τ. The energy content of the plasma at temperature T is 3nkT, where k is the Boltzmann constant. The rate of energy loss by mechanisms other than bremsstrahlung is thus simply 3nkT/τ. The energy balance of the plasma is the balance between the fusion energy heating the plasma and the energy-loss rate, which is the sum of 3nkT/τ and the bremsstrahlung. The condition satisfying this balance is called the ignition condition. An equation relates the product of density and energy confinement time, denoted nτ, to a function that depends only on the plasma temperature and the type of fusion reaction. For example, when the plasma is composed of deuterium and tritium, the smallest value of nτ required to achieve ignition is about 2×1020 particles per cubic metre times seconds, and the required temperature corresponds to an energy of about 25,000 eV. If the only energy losses are due to bremsstrahlung escaping from the plasma (meaning τ is infinite), the ignition temperature decreases to an energy level of 4,400 eV. Hence, the keys to generating usable amounts of fusion energy are to attain a sufficient plasma temperature and a sufficient confinement quality, as measured by the product nτ. At a temperature equivalent to 10,000 eV, the nτ product must be about 3×1020 particles per cubic metre times seconds.
Magnetic fusion energy generally creates plasmas with a density of about 3×1020 particles per cubic metre, which is about 10−8 of normal density. Hence, the characteristic time for heat to escape must be greater than about one second. This is a measure of the required degree of magnetic insulation for the heat content. Under these conditions the plasma remains in energy balance and can operate continuously if the ash of the nuclear fusion, namely helium, is removed (otherwise it will quench the plasma) and fuel is replenished.
ICF creates plasmas of much higher density, generally between 1031 and 1032 particles per cubic metre, or 1,000 to 10,000 times the normal density. As such, the confinement time, or minimum burn time, can be as short as 20×10−12 second. The objective in ICF is to achieve a temperature equivalent of 4,400 eV at the centre of the highly compressed fuel mass, while still having sufficient mass left around the centre so that the disassembly time will exceed the minimum burn time.
History of fusion energy research
The fusion process has been studied in order to understand nuclear matter and forces, to learn more about the nuclear physics of stellar objects, and to develop thermonuclear weapons. During the late 1940s and early '50s, research programs in the United States, United Kingdom, and the Soviet Union began to yield a better understanding of nuclear fusion, and investigators embarked on ways of exploiting the process for practical energy production. fusion reactor research focused primarily on using magnetic fields and electromagnetic forces to contain the extremely hot plasmas needed for thermonuclear fusion.
Researchers soon found, however, that it is exceedingly difficult to contain plasmas at fusion reaction temperatures because the hot gases tend to expand and escape from the enclosing magnetic structure. Plasma physics theory in the 1950s was incapable of describing the behaviour of the plasmas in many of the early magnetic confinement systems.
The undeniable potential benefits of practical fusion energy led to an increasing call for international cooperation. American, British, and Soviet fusion programs were strictly classified until 1958, when most of their research programs were made public at the Second Geneva Conference on the Peaceful Uses of Atomic Energy, sponsored by the United Nations. Since that time, fusion research has been characterized by international collaboration. In addition, scientists have also continued to study and measure fusion reactions between the lighter elements so as to arrive at a more accurate determination of reaction rates. The formulas developed by nuclear physicists for predicting the rate of fusion energy generation have been adopted by astrophysicists to derive new information about the structure and evolution of stars.
Work on the other major approach to fusion energy, inertial confinement fusion (ICF), was begun in the early 1960s. The initial idea was proposed in 1961, only a year after the reported invention of the laser, in a then-classified proposal to employ large pulses of laser energy (which no one then quite knew how to achieve) to implode and shock-heat matter to temperatures at which nuclear fusion would proceed vigorously. Aspects of inertial confinement fusion were declassified in the 1970s and, especially, in the early 1990s to reveal important aspects of the design of the targets containing fusion fuels. Very painstaking and sophisticated work to design and develop short-pulse, high-power lasers and suitable millimetre-sized targets continues, and significant progress has been made.
Although practical fusion reactors have not been built yet, the necessary conditions of plasma temperature and heat insulation have been largely achieved, suggesting that fusion energy for electric-power production is now a serious possibility. Commercial fusion reactors promise an inexhaustible source of electricity for countries worldwide. From a practical viewpoint, however, the initiation of nuclear fusion in a hot plasma is but the first step in a whole sequence of steps required to convert fusion energy to electricity. In the end, successful fusion power systems must be capable of producing electricity safely and in a cost-effective manner, with a minimum of radioactive waste and environmental impact. The quest for practical fusion energy remains one of the great scientific and engineering challenges of humankind.
Additional Reading
Further information can be found in Donald D. Clayton, Principles of Stellar Evolution and Nucleosynthesis (1968, reprinted 1983), a description of nuclear astrophysics covering energy generation and transport in stars, thermonuclear fusion reactions, and star burning; Francis F. Chen, Plasma Physics, 2nd ed. (1984), vol. 1 of Introduction to Plasma Physics and Controlled Fusion, a basic introduction; V.E. Golant, A.P. Zhilinsky, and I.E. Sakharov, Fundamentals of Plasma Physics (1980; originally published in Russian, 1977), an advanced text; J. Raeder et al., Controlled Nuclear Fusion: Fundamentals of Its Utilization for Energy Supply (1986; originally published in German, 1981), an introduction to fusion energy, its technology, and the engineering aspects of conceptual fusion power reactors; Robert W. Conn, “The Engineering of Magnetic Fusion Reactors,” Scientific American, 249(4):60–71 (October 1983), a descriptive article on the technology of fusion machines and future fusion-energy reactors; and Robert A. Gross, Fusion Energy (1984), an introductory text to fusion energy physics and technology, with an emphasis on the magnetic confinement fusion approach.
- assembled gem
- Assemblies of God
- assembly
- assembly language
- assembly line
- assemblé
- Assen
- Asser
- Asser, Tobias Michael Carel
- assessment
- assessor
- Assia Djebar
- assignat
- assigned counsel
- assimilation
- Assiniboia
- Assiniboin
- Assiniboine River
- Assisi
- assistance, writ of
- assize
- Assize of Clarendon
- Assize of Northampton
- Assizes of Jerusalem
- Associated Press