optical ceramics
Introduction
advanced industrial materials developed for use in optical applications.
Optical materials derive their utility from their response to infrared, optical, and ultraviolet light. The most obvious optical materials are glasses, which are described in the article industrial glass, but ceramics also have been developed for a number of optical applications. This article surveys several of these applications, both passive (e.g., windows, radomes, lamp envelopes, pigments) and active (e.g., phosphors, lasers, electro-optical components).
Passive devices
Optical and infrared windows
In their pure state, most ceramics are wide-band-gap insulators. This means that there is a large gap of forbidden states between the energy of the highest filled electron levels and the energy of the next highest unoccupied level. If this band gap is larger than optical light energies, these ceramics will be optically transparent (although powders and porous compacts of such ceramics will be white and opaque due to light scattering). Two applications of optically transparent ceramics are windows for bar-code readers at supermarkets and infrared radome and laser windows.
sapphire (a single-crystal form of aluminum oxide, Al2O3) has been used for supermarket checkout windows. It combines optical transparency with high scratch resistance. Similarly, single-crystal or infrared-transparent polycrystalline ceramics such as sodium chloride (salt) (NaCl), rubidium-doped potassium chloride (KCl), calcium fluoride (CaF), and strontium fluoride (SrF2) have been used for erosion-resistant infrared radomes, windows for infrared detectors, and infrared laser windows. These polycrystalline halide materials tend to transmit lower wavelengths than oxides, extending down to the infrared region; however, their grain boundaries and porosity scatter radiation. Therefore, they are best used as single crystals. As such, however, halides are insufficiently strong for large windows: they can plastically deform under their own weight. In order to strengthen them, single crystals are typically hot-forged to induce clean grain boundaries and large grain sizes, which do not decrease infrared transmission significantly but allow the body to resist deformation. Alternatively, large-grained material can be fusion-cast.
Lamp envelopes
 Electric discharge lamps (electric discharge lamp), in which enclosed gases are energized by an applied voltage and thereby made to glow, are extremely efficient light sources, but the heat and corrosion involved in their operation push optical ceramics to their thermochemical limits. A major breakthrough occurred in 1961, when Robert Coble of the General Electric Company in the United States demonstrated that alumina (a synthetic polycrystalline, Al2O3) could be sintered to optical density and translucency using magnesia (magnesium oxide, MgO) as a sintering aid. This technology permitted the extremely hot sodium discharge in the high-pressure sodium-vapour lamp to be contained in a refractory material that also transmitted its light (see Figure 1-->
Electric discharge lamps (electric discharge lamp), in which enclosed gases are energized by an applied voltage and thereby made to glow, are extremely efficient light sources, but the heat and corrosion involved in their operation push optical ceramics to their thermochemical limits. A major breakthrough occurred in 1961, when Robert Coble of the General Electric Company in the United States demonstrated that alumina (a synthetic polycrystalline, Al2O3) could be sintered to optical density and translucency using magnesia (magnesium oxide, MgO) as a sintering aid. This technology permitted the extremely hot sodium discharge in the high-pressure sodium-vapour lamp to be contained in a refractory material that also transmitted its light (see Figure 1--> ). The plasma within the inner alumina lamp envelope reaches temperatures of 1,200° C (2,200° F). Energy emission is within the yellow portion of the visible spectrum. The sodium-vapour lamp is nearly three times as efficient as its nearest competitor. It is now so common that the skylines of major cities are dominated by its amber glow.
). The plasma within the inner alumina lamp envelope reaches temperatures of 1,200° C (2,200° F). Energy emission is within the yellow portion of the visible spectrum. The sodium-vapour lamp is nearly three times as efficient as its nearest competitor. It is now so common that the skylines of major cities are dominated by its amber glow.Pigments (pigment)
The ceramic colour or pigment industry is a long-standing, traditional industry. Ceramic pigments or stains are made of oxide or selenide compounds in combination with specific transition-metal or rare-earth elements. Absorption of certain wavelengths of light by these species imparts specific colours to the compound. For example, cobalt aluminate (CoAl2O4) and cobalt silicate (Co2SiO4) are blue; tin-vanadium oxide (known as V-doped SnO2) and zirconium-vanadium oxide (V-doped ZrO2) are yellow; cobalt chromite (CoCr2O3) and chromium garnet (2CaO · Cr2O3 · 3SiO2) are green; and chromium hematite (CrFe2O3) is black. A true red colour, unavailable in naturally occurring silicate materials, is found in solid solutions of cadmium sulfide and cadmium selenide (CdS-CdSe).
Powdered pigments are incorporated into ceramic bodies or glazes in order to impart colour to the fired ware. Thermal stability and chemical inertness during firing are important considerations.
Active devices
Phosphors (phosphor)
Ceramic phosphors are employed for both general lighting (as in fluorescent lights) and for electronic imaging (as in cathode-ray tubes). Phosphors function when electrons within them are stimulated from stable, low-energy positions to higher levels by an appropriate means—e.g., thermal, optical, X-ray, or electron excitation. When the energized electrons drop back to lower energy levels, light can be emitted at one or more characteristic wavelengths. These wavelengths are determined by controlled dopants, referred to as activators. Examples of activated phosphors (and their resulting colour emissions) are lead-activated calcium tungstate (blue), manganese-activated zircon (green), lead- or manganese-activated calcium silicate (yellow to orange), and europium-activated yttrium vanadate (red). There are countless other examples.
Two major applications of phosphor ceramics are in cathode-ray tubes (cathode-ray tube) (CRTs) for television sets and computer monitors. Thin layers of phosphor powders are applied to the inside of the display screen of the CRT. Electrons are accelerated from the cathode toward the screen, directed by magnetic coils. Light emission (phosphorescence) occurs wherever the electron beam strikes the phosphor layer, and images are formed by high-speed scanning of the electron beam over the surface of the screen. Colour screens employ interspersed small dots of phosphors of each of the three primary colours (red, yellow, and blue), with separate electron beams to address each colour.
Efficient indoor lighting is usually accomplished by fluorescent lamps (fluorescent lamp). Phosphors of a suitably doped calcium halophosphate are deposited as thin powder layers on the inner surfaces of thin-walled glass tubes. The tubes are evacuated and backfilled with a mixture of mercury vapour and an inert gas. An electric discharge through the gas causes the mercury vapour to emit energy in the ultraviolet range, which strikes the phosphor layer and stimulates visible light emission. The resulting combination of blue and orange emission is comparable to that of incandescent lamps.
Phosphors must be manufactured by clean-room methods in order to eliminate unwanted impurities that can “kill” phosphorescence.
Lasers (laser)
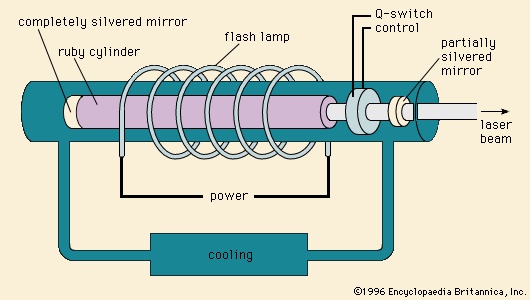 Lasing, or “light amplification by stimulated emission of radiation,” takes place in various media, including glasses and single-crystal ceramics. The first laser, operated by Theodore H. Maiman in 1960, consisted of a rod of synthetic ruby (single-crystal Al2O3 doped with chromium) that was excited by a flash lamp. (See Figure 2-->
Lasing, or “light amplification by stimulated emission of radiation,” takes place in various media, including glasses and single-crystal ceramics. The first laser, operated by Theodore H. Maiman in 1960, consisted of a rod of synthetic ruby (single-crystal Al2O3 doped with chromium) that was excited by a flash lamp. (See Figure 2--> .) Excitation, or pumping, involves promoting electrons within the dopant centres to higher energy levels by optical or electronic means. The decay of the stimulated electron to a lower energy state yields emission of light, which is contained within the lasing solid between two mirrors (one completely silvered and one partially silvered). As the emitted light reflects back and forth, it stimulates other centres until an intense, coherent, narrow beam of monochromatic light is released. Two well-known ceramic lasing materials are the chromium-doped Al2O3 known as ruby and a neodymium-doped yttrium aluminum garnet known as Nd-YAG.
.) Excitation, or pumping, involves promoting electrons within the dopant centres to higher energy levels by optical or electronic means. The decay of the stimulated electron to a lower energy state yields emission of light, which is contained within the lasing solid between two mirrors (one completely silvered and one partially silvered). As the emitted light reflects back and forth, it stimulates other centres until an intense, coherent, narrow beam of monochromatic light is released. Two well-known ceramic lasing materials are the chromium-doped Al2O3 known as ruby and a neodymium-doped yttrium aluminum garnet known as Nd-YAG.Electro-optical components
Electro-optical ceramics are materials that combine optical transparency with voltage-variable optical, or electro-optical (EO), behaviour. Single-crystal EO materials include lithium niobate (LiNbO3) and lithium tantalate (LiTaO3); polycrystalline EO materials include a lanthanum-modified lead zirconate tantalate known as PLZT. Among other EO properties, these materials exhibit voltage-dependent birefringence. Birefringence is the difference between the refractive index parallel to the optical axis of the crystal and the refractive index perpendicular to the optical axis. Because the propagation velocity is different in the two directions, a phase shift occurs, and this phase shift can be varied by an applied voltage. Such EO behaviour is the basis of a number of optical devices, including switches, modulators, and demodulators for high-speed optical communications. EO ceramic thin films also can be integrated with silicon semiconductors in so-called optoelectronic integrated circuits (OEICs).
 Optical ceramics constitute only one of several types of electroceramics. For a survey of all advanced electromagnetic applications, see electroceramics. For a directory to all the articles covering both traditional and advanced ceramics, see Industrial Ceramics: Outline of Coverage-->
Optical ceramics constitute only one of several types of electroceramics. For a survey of all advanced electromagnetic applications, see electroceramics. For a directory to all the articles covering both traditional and advanced ceramics, see Industrial Ceramics: Outline of Coverage--> .
.Additional Reading
Materials on optical ceramics may be found in A.J. Moulson and J.M. Herbert, Electroceramics: Materials, Properties, Applications (1990); Larry L. Hench and J.K. West, Principles of Electronic Ceramics (1990); and the section titled “Electrical/Electronic Applications for Advanced Ceramics,” in Theodore J. Reinhart (ed.), Engineered Materials Handbook, vol. 4, Ceramics and Glasses, ed. by Samuel J. Schneider (1991), pp. 1105–66.A good introduction to ceramics in general is provided by David W. Richerson, Modern Ceramic Engineering: Properties, Processing, and Use in Design, 2nd ed., rev. and expanded (1992). The processing of both traditional and advanced ceramics is described in James S. Reed, Introduction to the Principles of Ceramic Processing (1988); I.J. McColm and N.J. Clark, Forming, Shaping, and Working of High Performance Ceramics (1988); George Y. Onoda, Jr., and Larry L. Hench, Ceramic Processing Before Firing (1978); and four sections of the Reinhart book cited above: “Ceramic Powders and Processing,” pp. 41–122; “Forming and Predensification, and Nontraditional Densification Processes,” pp. 123–241; “Firing/Sintering: Densification,” pp. 242–312; and “Final Shaping and Surface Finishing,” pp. 313–376.
- Cambridge, Richard Owen
- Cambridgeshire
- Cambridge, University of
- Cambyses I
- Cambyses II
- Camden
- Camden, Battle of
- Camden, Charles Pratt, 1st Earl, Viscount Bayham Of Bayham Abbey, Baron Camden Of Camden Place
- Camden, John Jeffreys Pratt, 1st Marquess, 2nd Earl Camden, Earl Of The County Of Brecknock, Viscount Bayham Of Bayham Abbey, Baron Camden Of Camden Place
- Camden Town Group
- Camden, William
- camel
- camel hair
- Camellia
- Camelops
- Camelot
- camel racing
- Camembert cheese
- Camenae
- cameo
- cameo glass
- camera
- camera lucida
- camera obscura
- Camerarius, Joachim