osmium
chemical element
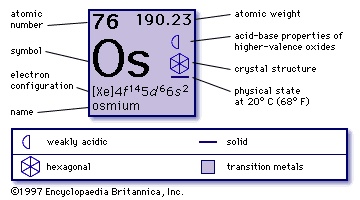 chemical element, one of the platinum metals (platinum group) of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table and the densest naturally occurring element. A gray-white metal, osmium is very hard, brittle, and difficult to work, even at high temperatures. Of the platinum metals it has the highest melting point, so fusing and casting are difficult. Osmium wires were used for filaments of early incandescent lamps before the introduction of tungsten. It has been used chiefly as a hardener in alloys of the platinum metals, though ruthenium has generally replaced it. A hard alloy of osmium and iridium has been used for tips of fountain pens and phonograph needles, and osmium tetroxide is used in certain organic syntheses.
chemical element, one of the platinum metals (platinum group) of Groups 8–10 (VIIIb), Periods 5 and 6, of the periodic table and the densest naturally occurring element. A gray-white metal, osmium is very hard, brittle, and difficult to work, even at high temperatures. Of the platinum metals it has the highest melting point, so fusing and casting are difficult. Osmium wires were used for filaments of early incandescent lamps before the introduction of tungsten. It has been used chiefly as a hardener in alloys of the platinum metals, though ruthenium has generally replaced it. A hard alloy of osmium and iridium has been used for tips of fountain pens and phonograph needles, and osmium tetroxide is used in certain organic syntheses.Pure osmium metal does not occur in nature. Osmium has a low crustal abundance of about 0.001 part per million. Though rare, osmium is found in native alloys with other platinum metals: in siserskite (up to 80 percent), in iridosmine (q.v.), in aurosmiridium (25 percent), and in slight amounts in native platinum. Processes for isolating it are an integral part of the metallurgical art that applies to all platinum metals.
The English chemist Smithson Tennant discovered the element together with iridium in the residues of platinum ores not soluble in aqua regia. He announced its isolation (1804) and named it for the unpleasant odour of some of its compounds (Greek osme, odour).
Of the platinum metals, osmium is the most rapidly attacked by air. The powdered metal, even at room temperature, exudes the characteristic odour of the poisonous, volatile tetroxide, OsO4. Because solutions of OsO4 are reduced to the black dioxide, OsO2, by some biological materials, it is sometimes used to stain tissues for microscopic examinations.
Osmium is, with ruthenium, the most noble of the platinum metals, and cold and hot acids are without effect on them. It can be dissolved by fused alkalies, especially if an oxidizing agent such as sodium chlorate is present. Osmium will react at 200° C with air or oxygen to form OsO4.
Osmium exhibits oxidation states from 0 to +8 in its compounds, with the exception of +1; well-characterized and stable compounds contain the element in +2, +3, +4, +6, and +8 states. There are also carbonyl and organometallic compounds in the low oxidation states −2, 0, and +1. Ruthenium is the only other element known to have an oxidation state of 8. (The chemistries of ruthenium and osmium are generally similar.) All compounds of osmium are easily reduced or decomposed by heating to form the free element as a powder or sponge. There is an extensive chemistry of the tetroxides, oxohalides, and oxo anions. There is little, if any, evidence that simple aquo ions exist, and virtually all their aqueous solutions, whatever the anions present, may be considered to contain complexes.
Natural osmium consists of a mixture of seven stable isotopes: osmium-184 (0.02 percent), osmium-186 (1.58 percent), osmium-187 (1.6 percent), osmium-188 (13.3 percent), osmium-189 (16.1 percent), osmium-190 (26.4 percent), osmium-192 (41.0 percent).
atomic number
76
atomic weight
190.2
melting point
3,000° C (5,432° F)
boiling point
about 5,000° C (9,032° F)
specific gravity
22.48 (20° C)
oxidation states
+2, +3, +4, +6, +8
electronic config.
【Xe】4f 145d66s2