borane
chemical compound
Introduction
 any of a homologous series of inorganic compounds of boron and hydrogen or their derivatives.
any of a homologous series of inorganic compounds of boron and hydrogen or their derivatives.The boron hydrides were first systematically synthesized and characterized during the period 1912 to roughly 1937 by the German chemist Alfred Stock. He called them boranes in analogy to the alkanes (saturated hydrocarbons (hydrocarbon)), the hydrides (hydride) of carbon (C), which is the neighbour of boron in the periodic table. Because the lighter boranes were volatile, sensitive to air and moisture, and toxic, Stock developed high-vacuum methods and apparatus for studying them. American work on boranes began in 1931, carried out by Hermann I. Schlesinger and Anton B. Burg. Boranes remained primarily of academic interest until World War II, when the U.S. government supported research to find volatile uranium compounds (borohydrides) for isotope separation, and the 1950s, when it supported programs to develop high-energy fuels for rockets and jet aircraft. (Boranes and their derivatives have much higher heats of combustion than hydrocarbon fuels.) William Nunn Lipscomb (Lipscomb, William Nunn, Jr.), Jr., received the 1976 Nobel Prize for Chemistry “for his studies on the structure of boranes illuminating problems of chemical bonding,” while one of Schlesinger's students, Herbert Charles Brown (Brown, Herbert Charles), shared the 1979 prize for his hydroboration reaction (1956), the remarkably easy addition of BH3 (in the form of BH3 · S) to unsaturated organic compounds (i.e., alkenes and alkynes) in ether solvents (S) at room temperature to yield organoboranes quantitatively (that is, in a reaction that proceeds wholly, or almost wholly, to completion). The hydroboration reaction in turn opened up new avenues of research in the area of stereospecific organic synthesis.
Physical properties of some common boranes Physical properties of some common boranesThe boranes that were prepared by Stock had the general composition BnHn + 4 and BnHn + 6, but more complex species, both neutral and negative (anionic (anion)), are known. The hydrides of boron are more numerous than those of any other element except carbon. The simplest isolable borane is B2H6, diborane(6). (The Arabic numeral in parentheses indicates the number of hydrogen atoms.) It is one of the most extensively studied and most synthetically useful chemical intermediates. It is commercially available, and for years many boranes and their derivatives were prepared from it, either directly or indirectly. Free BH3 (and B3H7) are very unstable, but they can be isolated as stable adducts (addition products) with Lewis bases (electron-donor molecules)—e.g., BH3 · N(CH3)3. Boranes may be solids, liquids, or gases; in general, their melting and boiling points increase with increasing complexity and molecular weight.
Structure and bonding of boranes
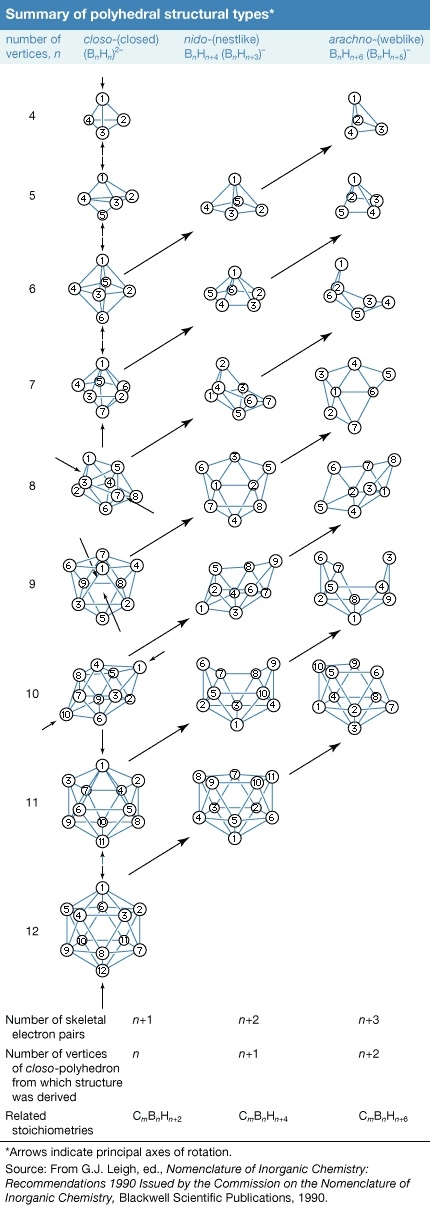 Rather than exhibiting the simple chain and ring configurations of carbon compounds, the boron atoms in the more complex boranes are located at the corners of polyhedrons, which can be considered either as deltahedrons (polyhedrons with triangular faces) or deltahedral fragments. Developing an understanding of these boron clusters has done much to help chemists rationalize the chemistry of other inorganic, organometallic, and transition-metal cluster compounds.
Rather than exhibiting the simple chain and ring configurations of carbon compounds, the boron atoms in the more complex boranes are located at the corners of polyhedrons, which can be considered either as deltahedrons (polyhedrons with triangular faces) or deltahedral fragments. Developing an understanding of these boron clusters has done much to help chemists rationalize the chemistry of other inorganic, organometallic, and transition-metal cluster compounds.One of several systems of nomenclature suggested by the International Union of Pure and Applied Chemistry (IUPAC) employs characteristic structural prefixes: (1) closo- (a corruption of “clovo,” from Latin clovis, meaning “cage”), deltahedrons of n boron atoms; (2) nido- (from Latin nidus, meaning “nest”), nonclosed structures in which the Bn cluster occupies n corners of an (n + 1)-cornered polyhedron—i.e., a closo-polyhedron with one missing vertex; (3) arachno- (Greek, meaning “spider's web”), clusters that are even more open, with boron atoms occupying n contiguous corners of an (n + 2)-cornered polyhedron—i.e., a closo-polyhedron with two missing vertices; (4) hypho- (Greek, meaning “to weave” or “a net”), the most open clusters, with boron atoms occupying n corners of an (n + 3)-cornered closo-polyhedron; and (5) klado- (Greek, meaning “branch”), n vertices of an n + 4-vertex closo-polyhedron occupied by n boron atoms. Members of the hypho- and klado- series are currently known only as borane derivatives. Linkage between two or more of these polyhedral borane clusters is indicated by the prefix conjuncto- (Latin, meaning “join together”). For example, conjuncto-B10H16 is produced by joining the B3H8 units from two B6H9 molecules via a B−B bond.
One of the reasons for the great interest in boranes is the fact that they possess structures different from any other class of compounds. Because the bonding in boranes involves multicentre bonding, in which three or more atoms share a pair of bonding electrons (electron), boranes are commonly called electron-deficient substances. Diborane(6) has the following structure:

This structure involves three-centre bridge bonding, in which one electron pair is shared between three (rather than two) atoms—two boron atoms and one hydrogen atom. (See chemical bonding: Advanced aspects of chemical bonding: Boranes (chemical bonding) for a discussion of the three-centre bond.) The ability of boron to form such bonds in addition to normal covalent bonds (covalent bond) leads to the formation of complex polyhedral boranes.
Reactions and synthesis of boranes
Although the simplest boranes—e.g., B2H6—are spontaneously flammable in air (burning with a characteristic green flame) and very reactive toward solvents containing replaceable protons (proton), reactivity generally decreases with increasing molecular weight. Some of the higher-molecular-weight polyhedral anions, such as B10H102− and B12H122−, are remarkably stable in air, water, and heat. Arachno-boranes are generally more reactive and less thermally stable than nido-boranes, which in turn are more reactive and less thermally stable than closo-boranes. Alfred Stock first obtained mixtures of boranes in low yield by treating magnesium boride (Mg3B2) with hydrochloric acid (HCl). Diborane is more easily prepared in high yield by reaction of iodine (I2) with sodium tetrahydroborate (NaBH4, commonly called sodium borohydride) in diglyme as a solvent,
2NaBH4 + I2 → B2H6(g) + 2NaI + H2(g),
or by reaction of a solid borohydride (i.e., a salt containing the BH4− ion) with an anhydrous acid,
2NaBH4 + 2H3PO4 → B2H6(g) + 2NaH2PO4 + 2H2(g).
The commercially available borohydrides, which are also important owing to their wide use as inorganic reducing agents, are the most commercially significant series of borane derivatives and the primary starting materials for preparing boranes on both a laboratory and an industrial scale. The stereospecific hydroboration reaction mentioned above,
3RCH=CH2 + 1/2B2H6 → B(CH2CH2R)3
(where R is an alkyl group), nearly quantitatively yields organoboranes, which in turn can yield various organic compounds such as the alkane corresponding to the initial alkene or the primary alcohol.
Additional Reading
Morris Srebnik, Valery M. Dembitsky, and Hijazi Abu Ali, Contemporary Aspects of Boron: Chemistry and Biological Applications (2005), is a survey on the preparation and applications of boron compounds; and N.N. Greenwood and A. Earnshaw, Chemistry of the Elements, 2nd. ed. (2006), covers the basic aspects of boron and the other chemical elements. Andrew Dequasie, The Green Flame: Surviving Government Secrecy (1991), provides a personal account of the U.S. military's projects to develop a boron-based fuel. Geoffrey Wilkinson, F. Gordon A. Stone, and Edward W. Abel (eds.), Comprehensive Organometallic Chemistry, 9 vol. (1982), contains several chapters dealing with carboranes and other heteroboranes; and Catherine E. Housecroft, Boranes and Metalloboranes (1990), includes basic information on these compounds, uses of physical methods to characterize them, and structural details about borane cages.
- Robert Stirling
- Roberts, Tom
- Robert Stone
- Robert Stroud
- Robert S. Woodworth
- Robert The Devil
- Robert the Strong
- Robert Todd Lincoln
- Robert Torrens
- Robert Treat Paine
- Robert Trimble
- Robert Venturi
- Robert Walpole, 1st earl of Orford
- Robert Whitehead
- Robert Wilhelm Bunsen
- Robert William Fogel
- Robert William Holley
- Robert Williams Wood
- Robert Winchelsey
- Robert Wise
- Robert Wood Johnson
- Robert Woodrow Wilson
- Robert W. Service
- Robert Young Hayne
- Roberval balance