carbon
chemical element
Introduction
 a nonmetallic chemical element in Group 14 (IVa) of the periodic table. Although widely distributed in nature, carbon is not particularly plentiful (it makes up only about 0.025 percent of the Earth's crust); yet it forms more compounds than all the other elements combined. In 1961 the isotope carbon-12 was selected to replace oxygen as the standard relative to which the atomic weights of all the other elements are measured; carbon-14, which is radioactive, is the isotope used in radiocarbon dating and radiolabeling.
a nonmetallic chemical element in Group 14 (IVa) of the periodic table. Although widely distributed in nature, carbon is not particularly plentiful (it makes up only about 0.025 percent of the Earth's crust); yet it forms more compounds than all the other elements combined. In 1961 the isotope carbon-12 was selected to replace oxygen as the standard relative to which the atomic weights of all the other elements are measured; carbon-14, which is radioactive, is the isotope used in radiocarbon dating and radiolabeling.Properties and uses
On a weight basis, carbon is 19th in order of elemental abundance in the crust of the Earth, and there are estimated to be 3.5 times as many carbon atoms as silicon atoms in the universe. Only hydrogen, helium, oxygen, neon, and nitrogen are atomically more abundant in the cosmos than carbon. Carbon is the cosmic product of the “burning” of helium in which three helium nuclei, atomic number 4, fuse to produce a carbon nucleus, atomic number 12.
In the crust of the Earth, elemental carbon is a minor component; however, carbon compounds (i.e., carbonates of magnesium and calcium) form common minerals (e.g., magnesite, dolomite, marble, or limestone). Coral and the shells of oysters and clams are primarily calcium carbonate. Carbon is widely distributed as coal and in the organic compounds that constitute petroleum, natural gas, and all plant and animal tissue. A natural sequence of chemical reactions called the carbon cycle—involving conversion of atmospheric carbon dioxide to carbohydrates by photosynthesis in plants, the consumption of these carbohydrates by animals and oxidation of them through metabolism to produce carbon dioxide and other products, and the return of carbon dioxide to the atmosphere—is one of the most important of all biological processes.
Carbon as an element was discovered by the first person to handle charcoal from fire; thus, together with sulfur, iron, tin, lead, copper, mercury, silver, and gold, carbon was one of the small group of elements well known in the ancient world. Modern carbon chemistry dates from the development of coals, petroleum, and natural gas as fuels and from the elucidation of synthetic organic chemistry, both substantially developed since the 1800s.
Elemental carbon exists in several forms, each of which has its own physical characteristics. Two of its well-defined forms, diamond and graphite, are crystalline in structure, but they differ in physical properties because the arrangements of the atoms in their structures are dissimilar. A third form, called fullerene, consists of a variety of molecules composed entirely of carbon. Yet another form, known as carbon black, is amorphous in structure and includes charcoal, lampblack, coal, and coke, although X-ray examination has revealed that these substances do possess a low degree of crystallinity. Diamond and graphite occur naturally on Earth, and they also can be produced synthetically; they are chemically inert but do combine with oxygen at high temperatures, just as amorphous carbon does. Fullerene was serendipitously discovered in 1985 as a synthetic product in the course of laboratory experiments to simulate the chemistry in the atmosphere of giant stars. They were later found to occur naturally in tiny amounts on Earth and in meteorites.
The word carbon probably derives from the Latin carbo, meaning variously “coal,” “charcoal,” “ember.” The term diamond, a corruption of the Greek word adamas, “the invincible,” aptly describes the permanence of this crystallized form of carbon, just as graphite, the name for the other crystal form of carbon, derived from the Greek verb graphein, “to write,” reflects its property of leaving a dark mark when rubbed on a surface. Before the discovery in 1779 that graphite when burned in air forms carbon dioxide, graphite was confused with both the metal lead and a superficially similar substance, the mineral molybdenite.
Pure diamond is the hardest naturally occurring substance known and is a poor conductor of electricity. Graphite, on the other hand, is a soft, slippery solid that is a good conductor of both heat and electricity. Carbon as diamond is the most expensive and brilliant of all the natural gemstones and also the hardest of abrasives. Graphite is used as a lubricant; in microcrystalline and nearly amorphous form, it is used as a black pigment, an adsorbent, a fuel, a filler for rubber, and, mixed with clay, as the “lead” of pencils. Because it conducts electricity but does not melt, graphite also is used for electrodes in electric furnaces and dry cells as well as for making crucibles in which metals are melted. Molecules of fullerene take the form of spheroidal closed cages containing various numbers of carbon atoms and long, hollow cylinders (the latter being known as nanotubes). They show promise in a range of applications, including high-tensile-strength materials, unique electronic and energy-storage devices, and safe encapsulation of flammable gases such as hydrogen. Elemental carbon is nontoxic.
Each of the amorphous forms of carbon has its own specific character; hence, each has its own particular applications. All are products of oxidation and other forms of decomposition of organic compounds. Coal and coke, for example, are used extensively as fuels; charcoal is used as an absorptive and filtering agent and as a fuel and in the manufacture of gunpowder. (Coals are elemental carbon mixed with varying amounts of carbon compounds; coke and charcoal are nearly pure carbon.) In addition to its uses in making inks, carbon paper, typewriter ribbons, and paints, carbon black also is added to the rubber used in tires to improve its wearing qualities. bone black, or animal charcoal, can adsorb gases and colouring matter from many other materials; a major use is in decolourizing raw sugar.
Carbon, either elemental or combined, is usually determined quantitatively by conversion to carbon dioxide gas, which can then be absorbed by other chemicals to give either a weighable product or a solution with acidic properties that can be titrated.
Production of elemental carbon
Until 1955, all diamonds were obtained from natural deposits, most significant in southern Africa but occurring also in Brazil, Venezuela, British Guiana (now Republic of Guyana), and Siberia. The single known source in the United States, in Arkansas, has no commercial importance, nor is India, once historically a source of fine diamonds, a significant present-day supplier. The primary source of diamonds is a soft, bluish-coloured peridotic rock called kimberlite (after the famous deposit at Kimberley, South Africa), found in volcanic structures called pipes; but many diamonds occur in alluvial deposits presumably resulting from the weathering of primary sources. Isolated finds around the world in regions where no sources are indicated have not been uncommon. Natural deposits are worked by crushing, by gravity and flotation separations, and by removal of diamonds by their adherence to a layer of grease on a suitable table. The following products result: (1) diamond proper—distorted cubic-crystalline, gem-quality stones varying from colourless to red, pink, blue, green, and yellow; (2) bort—minute, dark crystals of abrasive but not gem quality; (3) ballas—randomly oriented crystals of abrasive quality; (4) macles—triangular, pillow-shaped crystals that are industrially useful; and (5) carbonado—mixed diamond–graphite crystallites containing other impurities.
The successful laboratory conversion of graphite to diamond was made in 1955. The procedure involved the simultaneous use of extremely high pressure and temperature with iron as a solvent or catalyst. Subsequently, chromium, manganese, cobalt, nickel, and tantalum were substituted for iron. Synthetic diamonds are now manufactured in several countries and are being used increasingly in place of natural materials as industrial abrasives.
Graphite occurs naturally in many areas, the deposits of major importance being in South Korea, Austria, China, Mexico, Madagascar, Germany, Sri Lanka, and Russia. Both surface- and deep-mining techniques are used, followed by flotation, but the major portion of commercial graphite is produced by heating petroleum coke in an electric furnace. A better crystallized form, known as pyrolytic graphite, is obtained from the decomposition of low-molecular-weight hydrocarbons by heat. Graphite fibres of considerable tensile strength are obtained by carbonizing natural and synthetic organic fibres.
Amorphous carbon products are obtained by heating coal (to give coke), natural gas (to give blacks), or carbonaceous material of vegetable or animal origin, such as wood or bone (to give charcoal), at elevated temperatures in the presence of insufficient oxygen to allow combustion. The volatile by-products are recovered and used separately.
Structure of carbon allotropes
When an element exists in more than one crystalline form, those forms are called allotropes; the two most common allotropes of carbon are diamond and graphite. The crystal structure of diamond is an infinite three-dimensional array of carbon atoms, each of which forms a structure in which each of the bonds makes equal angles with its neighbours. If the ends of the bonds are connected, the structure is that of a tetrahedron, a three-sided pyramid of four faces (including the base). Every carbon atom is covalently bonded at the four corners of the tetrahedron to four other carbon atoms. The distance between carbon atoms along the bond is 1.54 × 10−8 centimetre, and this is called the single-bond length. The space lattice of the diamond can be visualized as carbon atoms in puckered hexagonal (six-sided) rings that lie roughly in one plane, the natural cleavage plane of the crystal; and these sheets of hexagonal, puckered rings are stacked in such a way that the atoms in every fourth layer lie in the same position as those in the first layer. The layer arrangement sequence is thus ABCABCA. . . . Such a crystal structure can be destroyed only by the rupture of many strong bonds: thus the extreme hardness, high sublimation temperature, the presumed extremely high melting point (extrapolated from known behaviour), and reduced chemical reactivity and insulating properties are all reasonable consequences of the crystal structure. Because of both the sense and the direction of the tetrahedral axis, four spatial orientations of carbon atoms exist, leading to two tetrahedral and two octahedral (eight-faced) forms of diamond.
The crystal structure of graphite amounts to a parallel stacking of layers of carbon atoms. Within each layer the carbon atoms lie in fused hexagonal rings that extend infinitely in two dimensions. The stacking pattern of the layers is ABABA . . . ; that is, each layer separates two identically oriented layers. Within each layer the carbon–carbon bond distance is 1.42 × 10−8 centimetre, which is intermediate between the single bond and double (1.33 × 10−8 centimetre) bond distances. All carbon–carbon bonds within a layer are the same (an observation that is interpreted in terms of complete π-bonding). The interlayer distance (3.37 × 10−8 centimetre) is sufficiently large to preclude localized bonding between the layers; the bonding between layers is probably by van der Waals interaction (i.e., the result of attraction between electrons of one carbon atom and the nuclei of neighbouring atoms). Ready cleavage, as compared with diamond, and electrical conductivity are consequences of the crystal structure of graphite. Other related properties are softness and lubricity (smoothness, slipperiness). A less common form of graphite, which occurs in nature, is based upon an ABCABCA . . . stacking, in which every fourth layer is the same. The amorphous varieties of carbon are based upon microcrystalline forms of graphite.
The greater degree of compactness in the diamond structure as compared with graphite suggests that by the application of sufficient pressure on graphite it should be converted to diamond. At room temperature and atmospheric pressure, diamond is actually less stable than graphite. The rate of conversion of diamond to graphite is so slow, however, that a diamond persists in its crystal form indefinitely. As temperature rises, the rate of conversion to graphite increases substantially, and at high temperatures it becomes (thermodynamically) favourable if the pressure is sufficiently high. At the same time, however, the rate of conversion decreases as the (thermodynamic) favourability increases. Thus, graphite does not yield diamond when heated under high pressure, and it appears that direct deformation of the graphite structure to the diamond structure in the solid state is not feasible. The occurrence of diamonds in iron–magnesium silicates in the volcanic structures called pipes and in iron–nickel and iron sulfide phases in meteorites suggests that they were formed by dissolution of carbon in those compounds and subsequent crystallization from them in the molten state at temperatures and pressures favourable to diamond stability. The successful synthesis of diamond is based upon this principle.
The crystal structure of graphite is of a kind that permits the formation of many compounds, called lamellar or intercalation compounds, by penetration of molecules or ions. Graphitic oxide and graphitic fluoride are nonconducting lamellar substances not obtained in true molecular forms that can be reproduced, but their formulas do approximate, respectively, the compositions of carbon dioxide and carbon monofluoride.
Nuclear properties
Carbon has two stable isotopes, carbon-12 (which makes up 98.89 percent of natural carbon) and carbon-13 (1.11 percent); 12 radioactive isotopes are known, of which the longest-lived is carbon-14, which has a half-life of 5,730 ± 40 years.
The notation used for the nucleus of atoms places the atomic mass as a presuperscript to the symbol of the element and the atomic number as a presubscript; thus, the isotope carbon-12 is symbolized 126C. Of the stable nuclides, the isotope carbon-13 is of particular interest in that its nuclear spin imparts response in a device called a nuclear magnetic resonance spectrometer, which is useful when investigating the molecular structures of covalently bonded compounds containing carbon. This isotope is also useful as a label in compounds that are to be analyzed by mass spectrometry, another device that is used extensively to identify atoms and molecules. Of the unstable nuclides, only carbon-14 is of sufficiently long half-life to be important. It is formed by the interaction of neutrons, produced by cosmic radiation, with nitrogen (N) in the atmosphere in a reaction that may be written as follows (neutron is symbolized as 10n, the nitrogen atom as 147N, and a hydrogen nucleus, or proton, as 11H):

The carbon-14 atoms from this reaction are converted to carbon dioxide by reaction with atmospheric oxygen and mixed and uniformly distributed with the carbon dioxide containing stable carbon-12. Living organisms use atmospheric carbon dioxide, whether with stable or radioactive carbon, through processes of photosynthesis and respiration, and thus their systems contain the constant ratio of carbon-12 to carbon-14 that exists in the atmosphere.
Death of an organism terminates this equilibration process; no fresh carbon dioxide is added to the dead substance. The carbon-14 present in the dead substance decays in accordance with its 5,730-year (± 40 years) half-life, while the carbon-12 remains what it was at death. Measurement of the carbon-14 activity at a given time thus allows calculation of the time elapsed after the death of the organism. Measurement of the carbon-14 activity in a cypress beam in the tomb of the Egyptian Pharaoh Snefru, for example, established the date of the tomb as c. 2600 BC. Many items of archaeological significance have been dated similarly.
The nuclides carbon-12 and carbon-13 are of importance in the carbon cycle of energy creation in certain stars. The cycle can be summarized in terms of nuclear equations, the separate steps being:
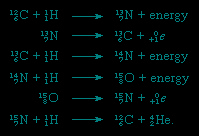
Summation of the equations allows the fusion process to be written as a reaction among four atoms of hydrogen to yield one atom of helium (He), two positrons (0+1e), and energy:

this equation does not show that the process uses up and regenerates the carbon-12. In a sense, carbon acts as a catalyst for this mode of converting mass to energy.
Compounds
More than 1,000,000 carbon compounds have been described in chemical literature, and chemists synthesize many new ones each year. Much of the diversity and complexity of organic forms is due to the capacity of carbon atoms for bonding with each other in various chain and ring structures and three-dimensional conformations, as well as for linking with other atoms. Indeed, carbon's compounds are so numerous, complex, and important that their study constitutes a specialized field of chemistry called organic chemistry, which derives its name from the fact that in the 19th century most of the then-known carbon compounds were considered to have originated in living organisms. (See chemical compound.)
All organic compounds, such as proteins, carbohydrates, and fats, contain carbon, and all plant and animal cells consist of carbon compounds and their polymers. (Polymers are macromolecules consisting of many simple molecules bonded together in specific ways.) With hydrogen, oxygen, nitrogen, and a few other elements, carbon forms compounds that make up about 18 percent of all the matter in living things. The processes by which organisms consume carbon and return it to their surroundings constitute the carbon cycle (q.v.).
Carbon is present as carbon dioxide in the Earth's atmosphere in amounts of about 0.03 percent by volume, and it is dissolved in all natural waters. Carbon occurs in the crust of the Earth in the form of carbonates in such rocks as marble, limestone, and chalk and in hydrocarbons—the principal constituents of coal, petroleum, and natural gas. Carbonate minerals are important sources of various metals, such as sodium, magnesium, calcium, copper, and lead.
At ordinary temperatures, carbon is very unreactive—it is difficult to oxidize—and it does not react with acids or alkalies. At high temperatures it combines with sulfur vapour to form carbon disulfide, with silicon and certain metals to form carbides, and with oxygen to form oxides, of which the most important are carbon monoxide, CO, and carbon dioxide, CO2. Because at high temperatures carbon combines readily with oxygen that is present in compounds with metals, large quantities of coke (an inexpensive form of carbon) are used in metallurgical processes to reduce (remove oxygen from) metal oxide ores, such as those of iron and zinc.
A type of chemical reaction in which one substance (an oxidizing agent) accepts electrons from another substance (a reducing agent) and is thereby reduced (while the reducing agent is oxidized) is frequently observed with carbon and its compounds. Although carbon is usually a reducing agent, under acidic conditions elemental carbon is a moderately strong oxidizing agent. The large energy of the carbon–carbon bond makes activation energy requirements for the reaction so high that direct reduction of carbon—e.g., to methane (formula CH4)—is impractical. Reduction of carbon monoxide to elemental carbon and oxidation of carbon monoxide to carbon dioxide are both feasible but impractical in solution. Under alkaline conditions, only the oxidation of formate ion (HCO2−) to carbonate ion (CO32−) is a reasonable process.
Carbon monoxide (CO) is both more readily absorbed and more firmly bound to the hemoglobin of the blood than is oxygen and is thus, even in small concentrations, a dangerous asphyxiant. Carbon dioxide (CO2) is an asphyxiant of significance only in relatively large concentrations; in small concentrations, it stimulates breathing. Hydrogen cyanide (HCN) and its derivatives (cyanogen compounds, cyanides) are all very toxic as protoplasmic poisons through the inhibition of tissue oxidation. Carbon tetrachloride (CCl4) and other chlorinated hydrocarbons damage the nervous system. Among organic compounds the most toxic are derivatives that contain the halogen elements (fluorine, chlorine, bromine, and iodine), sulfur, selenium, tellurium, nitrogen, phosphorus, arsenic, lead, and mercury. Most organometallic compounds are toxic, while oxygen-containing derivatives of the hydrocarbons are usually less toxic.
atomic number
6
atomic weight
12.011
melting point
3,550 °C (6,420 °F)
boiling point
4,827 °C (8,721 °F)
density
diamond
3.52 g/cm3
graphite
2.25 g/cm3
amorphous
1.9 g/cm3
oxidation states
+2, +3, +4
electron config.
1s22s22p2
- McGillivray, Alexander
- McGill, James
- McGill, Ralph
- McGill University
- McGinley, Phyllis
- McGovern, George S
- McGovern, Terry
- McGraw, John
- McGraw, Phil
- McGraw, Tim
- McGroarty, Sister Julia
- McGuane, Thomas
- McGuffey, William Holmes
- McGuire, Al
- McGwire, Mark
- McHenry, Robert
- Mchinji
- McIntire, Samuel
- McKay, Claude
- McKay, David O
- McKay, Donald
- McKean
- McKeesport
- McKellen, Sir Ian
- McKenna, Joseph