carbon dioxide
chemical compound
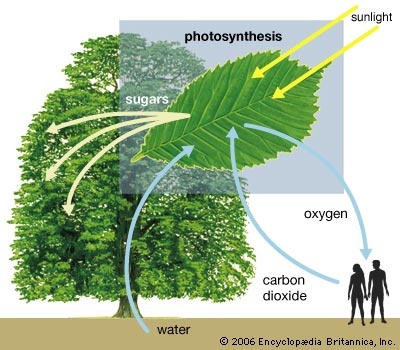 (CO2), a colourless gas having a faint, sharp odour and a sour taste; it is a minor component of the Earth's atmosphere (about 3 volumes in 10,000), formed in combustion of carbon-containing materials, in fermentation, and in respiration of animals and employed by plants in the photosynthesis of carbohydrates. The presence of the gas in the atmosphere keeps some of the radiant energy received by the Earth from being returned to space, thus producing the so-called greenhouse effect (q.v.). Industrially, it is recovered for numerous diverse applications from flue gases, as a by-product of the preparation of hydrogen for synthesis of ammonia, from limekilns, and from other sources.
(CO2), a colourless gas having a faint, sharp odour and a sour taste; it is a minor component of the Earth's atmosphere (about 3 volumes in 10,000), formed in combustion of carbon-containing materials, in fermentation, and in respiration of animals and employed by plants in the photosynthesis of carbohydrates. The presence of the gas in the atmosphere keeps some of the radiant energy received by the Earth from being returned to space, thus producing the so-called greenhouse effect (q.v.). Industrially, it is recovered for numerous diverse applications from flue gases, as a by-product of the preparation of hydrogen for synthesis of ammonia, from limekilns, and from other sources.Carbon dioxide was recognized as a gas different from others early in the 17th century by a Belgian chemist, Jan Baptist van Helmont, who observed it as a product of both fermentation and combustion. It liquefies upon compression to 75 kilograms per square centimetre (1,071 pounds per square inch) at 31° C (87.4° F) or to 16–24 kg per sq cm (230–345 lb per sq in.) at -23° to -12° C (-10° to 10° F). By the mid-20th century, most carbon dioxide was sold as the liquid. If the liquid is allowed to expand to atmospheric pressure, it cools and partially freezes to a snowlike solid called Dry Ice (q.v.) that sublimes (passes directly into vapour without melting) at -78.5° C (-109.3° F) at the pressure of the normal atmosphere.
At ordinary temperatures, carbon dioxide is quite unreactive; above 1,700° C (3,100° F) it partially decomposes into carbon monoxide and oxygen. Hydrogen or carbon also convert it to carbon monoxide at high temperatures. Ammonia reacts with carbon dioxide under pressure to form ammonium carbamate, then urea, an important component of fertilizers and plastics. Carbon dioxide is slightly soluble in water (1.79 volumes per volume at 0° C and atmospheric pressure, larger amounts at higher pressures), forming a weakly acidic solution. This solution contains the dibasic acid called carbonic acid (H2CO3).
Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages.
Ignited magnesium continues to burn in carbon dioxide, but the gas does not support the combustion of most materials. Prolonged exposure of humans to concentrations of 5 percent carbon dioxide may cause unconsciousness and death.
- conscription
- Conseil de l'Entente
- Conseil d'État
- consejo real
- Conselheiro Lafaiete
- conservation
- conservation law
- conservation of energy
- conservation of mass
- conservation of momentum
- conservatism
- Conservative Baptist Association of America
- conservative force
- Conservative Judaism
- Conservative Party
- Conservative Party of Canada
- conservatory
- consideration
- Considérant, Victor-Prosper
- consistory
- console
- Consolidated Rail Corporation
- Con Son
- consonance
- consonance and dissonance