tetrachloroethylene
chemical compound
also called perchloroethylene
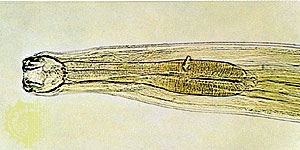 a colourless, dense, nonflammable, highly stable liquid belonging to the family of organic halogen compounds. Tetrachloroethylene is a powerful solvent for many organic substances. By the mid-20th century it had become the most widely used solvent in dry cleaning (displacing carbon tetrachloride and trichloroethylene) and was also commonly used for cleaning metal objects in vapour-degreasing apparatuses. Its broad acceptance has been based upon its nonflammability and low toxicity. Small quantities are also employed as a vermifuge, particularly against hookworm (see photograph-->
a colourless, dense, nonflammable, highly stable liquid belonging to the family of organic halogen compounds. Tetrachloroethylene is a powerful solvent for many organic substances. By the mid-20th century it had become the most widely used solvent in dry cleaning (displacing carbon tetrachloride and trichloroethylene) and was also commonly used for cleaning metal objects in vapour-degreasing apparatuses. Its broad acceptance has been based upon its nonflammability and low toxicity. Small quantities are also employed as a vermifuge, particularly against hookworm (see photograph--> ).
).Tetrachloroethylene was first prepared in 1821 by the English physicist Michael Faraday; (Faraday, Michael) it has been commercially manufactured since about 1910, mostly from trichloroethylene. Tetrachloroethylene is denser than water and practically insoluble in it.
- Atli, Lay of
- Atlixco
- Atlántico
- atman
- Atmore
- atmosphere
- atmosphere, evolution of
- atmosphere saffir-simpson
- atmosphere w n pacific
- Atmospheric abundances for Jupiter
- atmospheric circulation
- atmospheric corona
- atmospheric pressure
- atmospheric refraction
- atmospheric science
- atmospheric turbulence
- atoll
- atom
- Atom Egoyan
- atomic bomb
- atomic clock
- Atomic Energy Commission
- atomic mass
- atomic number
- atomic physics