lanthanum
chemical element
 (La), chemical element, rare-earth metal of transition Group IIIb of the periodic table, prototype of the lanthanoid series of elements. Lanthanum is a ductile and malleable, silvery-white metal, soft enough to be cut with a knife. The element was discovered as the oxide (lanthana) in 1839 by Carl Gustaf Mosander (Mosander, Carl Gustaf), who distinguished it from cerium oxide (ceria). Its name is derived from the Greek lanthanein, meaning “to be concealed,” indicating that it is difficult to isolate.
(La), chemical element, rare-earth metal of transition Group IIIb of the periodic table, prototype of the lanthanoid series of elements. Lanthanum is a ductile and malleable, silvery-white metal, soft enough to be cut with a knife. The element was discovered as the oxide (lanthana) in 1839 by Carl Gustaf Mosander (Mosander, Carl Gustaf), who distinguished it from cerium oxide (ceria). Its name is derived from the Greek lanthanein, meaning “to be concealed,” indicating that it is difficult to isolate.Lanthanum occurs in the rare-earth minerals monazite and bastnaesite. It is concentrated commercially by crystallization of ammonium lanthanum nitrate. Ion-exchange and solvent extraction methods are used when high purity is desired. The metal itself is prepared by electrolysis of fused anhydrous halides or by reduction of its halides by alkali or alkaline-earth metals (e.g., reduction of the fluoride with calcium). misch metal—used as cigarette-lighter flints, as a getter that removes traces of oxygen in electron tubes, and in metallurgy—is one-fourth lanthanum.
Lanthanum exhibits three allotropic (structural) forms. Two isotopes occur in nature: stable lanthanum-139 (99.911 percent) and very long-lived radioactive lanthanum-138 (0.089 percent). The isotope lanthanum-140 has been detected as a fission product in snow after nuclear-test explosions.
Lanthanum is the second most reactive of the rare-earth metals (europium is first); it rapidly tarnishes in dry air, ignites in air at 440° C (824° F), and reacts vigorously with hot water. In its compounds its only oxidation state is +3. The ionic radius is the largest of the rare-earth M3+ ions, and as a consequence the white oxide La2O3 is the most alkaline rare-earth oxide.
Highly purified lanthanum oxide is an ingredient in the manufacture of low-dispersion, high-refraction glasses for lens components. The technical grade fluoride is used as core material for arc-light carbons.
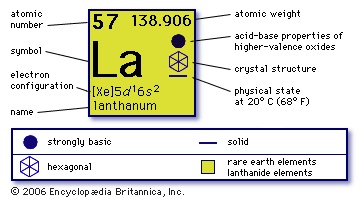
atomic number
57
atomic weight
138.91
melting point
920° C (1,688° F)
boiling point
3,454° C (6,249° F)
specific gravity
6.166 (25° C)
oxidation state
+3
electronic config.
【Xe】5d16s2
- Ackermann, Konrad Ernst
- Ackermann, Louise-Victorine
- Ackroyd, Peter
- Acmeist
- acne
- A.C. Nielsen
- Acoemeti
- acolyte
- Acoma
- Aconcagua, Mount
- Aconcagua River
- aconite
- Acontius
- Acontius, Jacobus
- Acorales
- acorn
- acorn and nut weevil
- acorn worm
- A Coruña
- acosmism
- Acosta, Joaquín
- Acosta, José de
- Acosta, Uriel
- acouchy
- acoustic impedance