technetium
chemical element
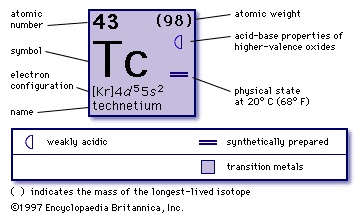 chemical element, synthetic radioactive metal of Group 7 (VIIb) of the periodic table, the first element to be artificially produced. The isotope technetium-97 (2,600,000-year half-life) was discovered (1937) by the Italian mineralogist Carlo Perrier and the Italian-born American physicist Emilio Segrè (Segrè, Emilio) in a sample of molybdenum that had been bombarded by deuterons in the Berkeley (California) cyclotron. This isotope is the longest-lived member of a set from technetium-92 to technetium-107 that has since been produced. The most important isotope, because it is the only one available on a large scale, is technetium-99 (212,000-year half-life); it is produced in kilogram quantities as a fission product in nuclear reactors. Technetium metal looks like platinum but is usually obtained as a gray powder. It crystallizes in the hexagonal close-packed structure and is a superconductor below 11.2 K. Except for technetium-99, technetium-97, and technetium-98 (1,500,000-year half-life), technetium isotopes are short-lived. Technetium has essentially no uses.
chemical element, synthetic radioactive metal of Group 7 (VIIb) of the periodic table, the first element to be artificially produced. The isotope technetium-97 (2,600,000-year half-life) was discovered (1937) by the Italian mineralogist Carlo Perrier and the Italian-born American physicist Emilio Segrè (Segrè, Emilio) in a sample of molybdenum that had been bombarded by deuterons in the Berkeley (California) cyclotron. This isotope is the longest-lived member of a set from technetium-92 to technetium-107 that has since been produced. The most important isotope, because it is the only one available on a large scale, is technetium-99 (212,000-year half-life); it is produced in kilogram quantities as a fission product in nuclear reactors. Technetium metal looks like platinum but is usually obtained as a gray powder. It crystallizes in the hexagonal close-packed structure and is a superconductor below 11.2 K. Except for technetium-99, technetium-97, and technetium-98 (1,500,000-year half-life), technetium isotopes are short-lived. Technetium has essentially no uses.Technetium occurs in the Earth's crust as minute traces from the spontaneous fission of uranium; the relatively short half-lives preclude the existence of any primordial technetium on Earth. The American astronomer Paul W. Merrill's discovery in 1952 that technetium-99 is present in S-type stars was a valuable piece of evidence concerning stellar evolution and nucleosynthesis. Technetium, chemically similar to rhenium (atomic number 75), exists in oxidation states of +7, +6, and +4 in compounds such as potassium pertechnetate, KTcO4, technetium chloride, TcCl6, and technetium sulfide, TcS2, respectively. Compounds are known in all formal oxidation states from −1 to +7.
atomic number
43
commonest isotope
(99)
melting point
2,172° C (3,942° F)
boiling point
4,877° C (8,811° F)
specific gravity
11.5 (20° C)
oxidation states
+4, +6, +7
electronic config.
【Kr】4d65s1
- polarization
- polar motion
- polarography
- Polaroid Corporation
- polaron
- polar wandering
- polder
- Poldervaart, Arie
- Polding, John Bede
- Poldi Pezzoli, Museo
- polecat
- Polemoniaceae
- polenta
- Polenta Family
- Pole of Inaccessibility
- Pole, Reginald
- Pole, Richard de la
- polestar
- pole vault
- Polevskoy
- Polgar, Judit
- Polgar, Zsuzsa
- Poliakoff, Serge
- police
- Police Gazette, The