Coordination numbers and geometries of metal cyanide complexes
Table
Coordination numbers and geometries of metal cyanide complexes
electron
configuration* metal ion cyanide
complex geometry total number of
valence electrons
d2 Mo4+ 【Mo(CN)8】4− dodecahedral 18
d3 Cr3+ 【Cr(CN)6】3− octahedral 15
d4 Mn3+ 【Mn(CN)6】3− octahedral 16
d5 Fe3+ 【Fe(CN)6】3− octahedral 17
d6 Co3+ 【Co(CN)6】3− octahedral 18
d7 Co2+ 【Co(CN)5】3− square pyramidal 17
d8 Ni2+ 【Ni(CN)4】2− square planar 16
d8 Ni2+ 【Ni(CN)5】3− square pyramidal or
trigonal bipyramidal 18
d10 Cd2+ 【Cd(CN)4】2− tetrahedral 18
d10 Ag+ 【Ag(CN)2】− linear 14
*Number of d electrons indicated by superscript.
See as table: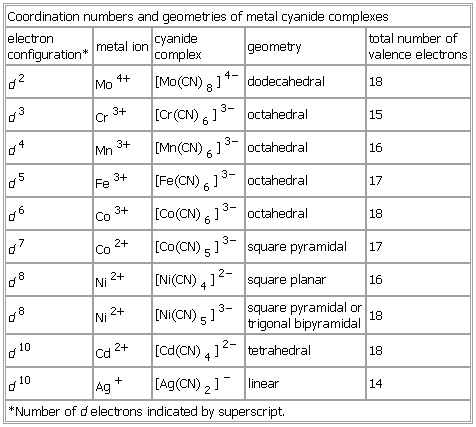

- World Literary Prizes 2006
- World Literary Prizes 2007
- World Literary Prizes 2008
- World Meteorological Organization
- World Methodist Council
- world music
- World of Warcraft
- World Production of Centrifugal Sugar, Table
- World Production of Centrifugal Sugar, Table 1
- World Production of Centrifugal Sugar, Table 2
- World Production of Centrifugal Sugar, Table 3
- World Production of Centrifugal Sugar, Table 4
- World Production of Crude Steel, Table
- World Production of Dairy Products, Table
- World Production of Major Oilseeds and Products, Table
- World Production of Major Oilseeds and Products, Table 1
- World Production of Major Oilseeds and Products, Table 2
- World Production of Major Oilseeds and Products, Table 3
- World Production of Milk1, Table
- World Production of Milk, Table
- World Production of Milk, Table 1
- World Production of Milk, Table 2
- World Production of Oilseeds and Products, Table
- World Production of Pig Iron, Table
- World professional snooker championship